Properties of CD34+ CML stem/progenitor cells that correlate with different clinical responses to imatinib mesylate
- PMID: 20574046
- PMCID: PMC2951856
- DOI: 10.1182/blood-2009-05-222471
Properties of CD34+ CML stem/progenitor cells that correlate with different clinical responses to imatinib mesylate
Abstract
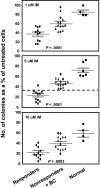
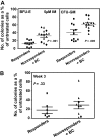
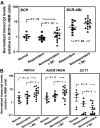
Imatinib mesylate (IM) induces clinical remissions in chronic-phase chronic myeloid leukemia (CML) patients but IM resistance remains a problem. We recently identified several features of CML CD34(+) stem/progenitor cells expected to confer resistance to BCR-ABL-targeted therapeutics. From a study of 25 initially chronic-phase patients, we now demonstrate that some, but not all, of these parameters correlate with subsequent clinical response to IM therapy. CD34(+) cells from the 14 IM nonresponders demonstrated greater resistance to IM than the 11 IM responders in colony-forming cell assays in vitro (P < .001) and direct sequencing of cloned transcripts from CD34(+) cells further revealed a higher incidence of BCR-ABL kinase domain mutations in the IM nonresponders (10%-40% vs 0%-20% in IM responders, P < .003). In contrast, CD34(+) cells from IM nonresponders and IM responders were not distinguished by differences in BCR-ABL or transporter gene expression. Interestingly, one BCR-ABL mutation (V304D), predicted to destabilize the interaction between p210(BCR-ABL) and IM, was detectable in 14 of 20 patients. T315I mutant CD34(+) cells found before IM treatment in 2 of 20 patients examined were preferentially amplified after IM treatment. Thus, 2 properties of pretreatment CML stem/progenitor cells correlate with subsequent response to IM therapy. Prospective assessment of these properties may allow improved patient management.
Figures


References
-
- Goldman JM, Melo JV. Chronic myeloid leukemia: advances in biology and new approaches to treatment. N Engl J Med. 2003;349(15):1451–1464. - PubMed
-
- Jiang X. Cancer Nanotechnology. Stevenson Ranch, CA: American Scientific Publishers; 2007. Molecular and cellular mechanisms of deregulated hematopoietic stem cell functions in chronic myeloid leukemia. pp. 135–157.
-
- Savona M, Talpaz M. Getting to the stem of chronic myeloid leukaemia. Nat Rev Cancer. 2008;8(5):341–350. - PubMed
-
- Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247(4946):1079–1082. - PubMed
-
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

