Low doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesis
- PMID: 20574535
- PMCID: PMC2888592
- DOI: 10.1371/journal.pone.0011222
Low doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesis
Abstract
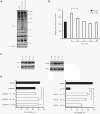
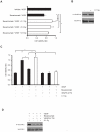
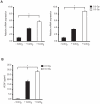
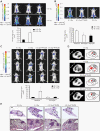
Radiotherapy is a widely used treatment option in cancer. However, recent evidence suggests that doses of ionizing radiation (IR) delivered inside the tumor target volume, during fractionated radiotherapy, can promote tumor invasion and metastasis. Furthermore, the tissues that surround the tumor area are also exposed to low doses of IR that are lower than those delivered inside the tumor mass, because external radiotherapy is delivered to the tumor through multiple radiation beams, in order to prevent damage of organs at risk. The biological effects of these low doses of IR on the healthy tissue surrounding the tumor area, and in particular on the vasculature remain largely to be determined. We found that doses of IR lower or equal to 0.8 Gy enhance endothelial cell migration without impinging on cell proliferation or survival. Moreover, we show that low-dose IR induces a rapid phosphorylation of several endothelial cell proteins, including the Vascular Endothelial Growth Factor (VEGF) Receptor-2 and induces VEGF production in hypoxia mimicking conditions. By activating the VEGF Receptor-2, low-dose IR enhances endothelial cell migration and prevents endothelial cell death promoted by an anti-angiogenic drug, bevacizumab. In addition, we observed that low-dose IR accelerates embryonic angiogenic sprouting during zebrafish development and promotes adult angiogenesis during zebrafish fin regeneration and in the murine Matrigel assay. Using murine experimental models of leukemia and orthotopic breast cancer, we show that low-dose IR promotes tumor growth and metastasis and that these effects were prevented by the administration of a VEGF receptor-tyrosine kinase inhibitor immediately before IR exposure. These findings demonstrate a new mechanism to the understanding of the potential pro-metastatic effect of IR and may provide a new rationale basis to the improvement of current radiotherapy protocols.
Conflict of interest statement
Figures



References
-
- Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. - PubMed
-
- Madani I, De Neve W, Mareel M. Does ionizing radiation stimulate cancer invasion and metastasis? Bull Cancer. 2008;95:292–300. - PubMed
-
- Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, et al. Inhibition of {alpha}v{beta}3 Integrin Survival Signaling Enhances Antiangiogenic and Antitumor Effects of Radiotherapy. Clin Cancer Res. 2005;11:6270–6279. - PubMed
-
- McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. - PubMed
-
- Nozue M, Isaka N, Fukao K. Over-expression of vascular endothelial growth factor after preoperative radiation therapy for rectal cancer. Oncol Rep. 2001;8:1247–1249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

