ADAMTS-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity
- PMID: 20574651
- PMCID: PMC11115784
- DOI: 10.1007/s00018-010-0431-6
ADAMTS-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity
Abstract
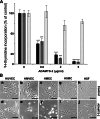
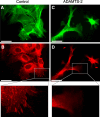
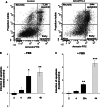
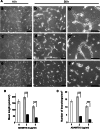
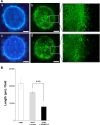
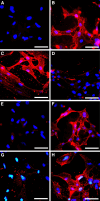
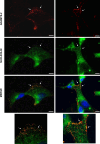
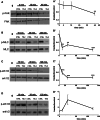
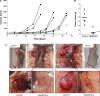
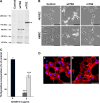
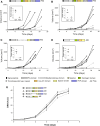
ADAMTS-2 is a metalloproteinase that plays a key role in the processing of fibrillar procollagen precursors into mature collagen molecules by excising the amino-propeptide. We demonstrate that recombinant ADAMTS-2 is also able to reduce proliferation of endothelial cells, and to induce their retraction and detachment from the substrate resulting in apoptosis. Dephosphorylation of Erk1/2 and MLC largely precedes the ADAMTS-2 induced morphological alterations. In 3-D culture models, ADAMTS-2 strongly reduced branching of capillary-like structures formed by endothelial cells and their long-term maintenance and inhibited vessels formation in embryoid bodies (EB). Growth and vascularization of tumors formed in nude mice by HEK 293-EBNA cells expressing ADAMTS-2 were drastically reduced. A similar anti-tumoral activity was observed when using cells expressing recombinant deleted forms of ADAMTS-2, including catalytically inactive enzyme. Nucleolin, a nuclear protein also found to be associated with the cell membrane, was identified as a potential receptor mediating the antiangiogenic properties of ADAMTS-2.
Figures

Similar articles
-
The procollagen N-proteinases ADAMTS2, 3 and 14 in pathophysiology.Matrix Biol. 2015 May-Jul;44-46:46-53. doi: 10.1016/j.matbio.2015.04.001. Epub 2015 Apr 8. Matrix Biol. 2015. PMID: 25863161 Review.
-
Anti-angiogenic properties of ADAMTS-4 in vitro.Int J Exp Pathol. 2012 Feb;93(1):70-7. doi: 10.1111/j.1365-2613.2011.00802.x. Int J Exp Pathol. 2012. PMID: 22264287 Free PMC article.
-
Interleukin-6 upregulates expression of ADAMTS-4 in fibroblast-like synoviocytes from patients with rheumatoid arthritis.Int J Rheum Dis. 2012 Feb;15(1):36-44. doi: 10.1111/j.1756-185X.2011.01656.x. Epub 2011 Sep 14. Int J Rheum Dis. 2012. PMID: 22324945
-
Transforming growth factor-beta induces secretion of activated ADAMTS-2. A procollagen III N-proteinase.J Biol Chem. 2003 May 23;278(21):19549-57. doi: 10.1074/jbc.M300767200. Epub 2003 Mar 19. J Biol Chem. 2003. PMID: 12646579
-
[Research progress of a disintegrin and metalloproteinase with thrombospondin motif 4 and 5 in osteoarthritis].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013 Sep;27(9):1080-4. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013. PMID: 24279019 Review. Chinese.
Cited by
-
The Function and Roles of ADAMTS-7 in Inflammatory Diseases.Mediators Inflamm. 2015;2015:801546. doi: 10.1155/2015/801546. Epub 2015 Nov 30. Mediators Inflamm. 2015. PMID: 26696755 Free PMC article. Review.
-
The roles of ADAMTS in angiogenesis and cancer.Tumour Biol. 2015 Jun;36(6):4039-51. doi: 10.1007/s13277-015-3461-8. Epub 2015 Apr 28. Tumour Biol. 2015. PMID: 25916206 Review.
-
ADAMTS6 suppresses tumor progression via the ERK signaling pathway and serves as a prognostic marker in human breast cancer.Oncotarget. 2016 Sep 20;7(38):61273-61283. doi: 10.18632/oncotarget.11341. Oncotarget. 2016. PMID: 27542224 Free PMC article.
-
Isthmin-A Multifaceted Protein Family.Cells. 2022 Dec 21;12(1):17. doi: 10.3390/cells12010017. Cells. 2022. PMID: 36611811 Free PMC article. Review.
-
A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease.Biochem Pharmacol. 2019 Jun;164:188-204. doi: 10.1016/j.bcp.2019.03.033. Epub 2019 Mar 21. Biochem Pharmacol. 2019. PMID: 30905657 Free PMC article. Review.
References
-
- Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim Biophys Acta. 2004;1654:13–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

