Thermally responsive injectable hydrogel incorporating methacrylate-polylactide for hydrolytic lability
- PMID: 20575552
- PMCID: PMC3208233
- DOI: 10.1021/bm1004299
Thermally responsive injectable hydrogel incorporating methacrylate-polylactide for hydrolytic lability
Abstract
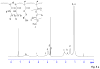
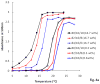
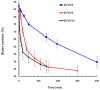
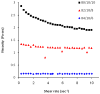
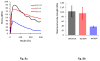
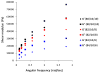
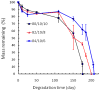
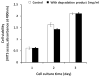
Injectable thermoresponsive hydrogels are of interest for a variety of biomedical applications, including regional tissue mechanical support as well as drug and cell delivery. Within this class of materials there is a need to provide options for gels with stronger mechanical properties as well as variable degradation profiles. To address this need, the hydrolytically labile monomer, methacrylate-polylactide (MAPLA), with an average 2.8 lactic acid units, was synthesized and copolymerized with N-isopropylacrylamide (NIPAAm) and 2-hydroxyethyl methacrylate (HEMA) to obtain bioabsorbable thermally responsive hydrogels. Poly(NIPAAm-co-HEMA-co-MAPLA) with three monomer feed ratios (84/10/6, 82/10/8, and 80/10/10) was synthesized and characterized with NMR, FTIR, and GPC. The copolymers were soluble in saline at reduced temperature (<10 degrees C), forming clear solutions that increased in viscosity with the MAPLA feed ratio. The copolymers underwent sol-gel transition at lower critical solution temperatures of 12.4, 14.0, and 16.2 degrees C, respectively, and solidified immediately upon being placed in a 37 degrees C water bath. The warmed hydrogels gradually excluded water to reach final water contents of approximately 45%. The hydrogels as formed were mechanically strong, with tensile strengths as high as 100 kPa and shear moduli of 60 kPa. All three hydrogels were completely degraded (solubilized) in PBS over a 6-7 month period at 37 degrees C, with a higher MAPLA feed ratio resulting in a faster degradation period. Culture of primary vascular smooth muscle cells with degradation solutions demonstrated a lack of cytotoxicity. The synthesized hydrogels provide new options for biomaterial injection therapy where increased mechanical strength and relatively slow resorption rates would be attractive.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

