Alkaline phosphatase from venom of the endoparasitoid wasp, Pteromalus puparum
- PMID: 20575745
- PMCID: PMC3014669
- DOI: 10.1673/031.010.1401
Alkaline phosphatase from venom of the endoparasitoid wasp, Pteromalus puparum
Abstract
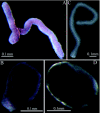
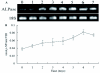
Using chromogenic substrates 5-bromo-4-chloro-3'-indolyl phosphate and nitro blue tetrazolium, alkaline phosphatase (ALPase) was histochemically detected in the venom apparatus of an endoparasitoid wasp, Pteromalus puparum L. (Hymenoptera: Pteromalidae). Ultrastructural observations demonstrated its presence in the secretory vesicles and nuclei of the venom gland secretory cells. Using p-nitrophenyl phosphate as substrate to measure enzyme activity, the venom ALPase was found to be temperature dependent with bivalent cation effects. The full-length cDNA sequence of ALPase was amplified from the cDNA library of the venom apparatus of P. puparum, providing the first molecular characterization of ALPase in the venom of a parasitoid wasp. The cDNA consisted of 2645 bp with a 1623 bp open reading frame coding for 541 deduced amino acids with a predicted molecular mass of 59.83 kDa and pI of 6.98. Using multiple sequence alignment, the deduced amino acid sequence shared high identity to its counterparts from other insects. A signal peptide and a long conserved ALPase gene family signature sequence were observed. The amino acid sequence of this venom protein was characterized with different potential glycosylation, myristoylation, phosphorylation sites and metal ligand sites. The transcript of the ALPase gene was detected by RT-PCR in the venom apparatus with development related expression after adult wasp emergence, suggesting a possible correlation with the oviposition process.
Figures

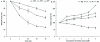

Similar articles
-
A Venom Gland Extracellular Chitin-Binding-Like Protein from Pupal Endoparasitoid Wasps, Pteromalus Puparum, Selectively Binds Chitin.Toxins (Basel). 2015 Nov 30;7(12):5098-113. doi: 10.3390/toxins7124867. Toxins (Basel). 2015. PMID: 26633500 Free PMC article.
-
Molecular cloning and characterization of acid phosphatase in venom of the endoparasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae).Toxicon. 2008 Jun 15;51(8):1391-9. doi: 10.1016/j.toxicon.2008.03.008. Epub 2008 Mar 18. Toxicon. 2008. PMID: 18448143
-
Proteomic analysis of the venom from the endoparasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae).Arch Insect Biochem Physiol. 2010 Sep;75(1):28-44. doi: 10.1002/arch.20380. Arch Insect Biochem Physiol. 2010. PMID: 20648599
-
Expression of immune-response genes in lepidopteran host is suppressed by venom from an endoparasitoid, Pteromalus puparum.BMC Genomics. 2010 Sep 2;11:484. doi: 10.1186/1471-2164-11-484. BMC Genomics. 2010. PMID: 20813030 Free PMC article.
-
Venom of the parasitoid wasp Pteromalus puparum contains an odorant binding protein.Arch Insect Biochem Physiol. 2015 Feb;88(2):101-10. doi: 10.1002/arch.21206. Epub 2014 Sep 25. Arch Insect Biochem Physiol. 2015. PMID: 25256903
Cited by
-
A Venom Gland Extracellular Chitin-Binding-Like Protein from Pupal Endoparasitoid Wasps, Pteromalus Puparum, Selectively Binds Chitin.Toxins (Basel). 2015 Nov 30;7(12):5098-113. doi: 10.3390/toxins7124867. Toxins (Basel). 2015. PMID: 26633500 Free PMC article.
-
Comparative Analysis of the Venom Proteins from Two Eupelmid Egg Parasitoids Anastatus japonicus and Mesocomys trabalae.Biology (Basel). 2023 May 10;12(5):700. doi: 10.3390/biology12050700. Biology (Basel). 2023. PMID: 37237513 Free PMC article.
References
-
- Abt M, Rivers DB. Characterization of phenoloxidase activity in venom from the ectoparastioid Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae). Journal of Invertebrte Pathology. 2007;94:108–118. - PubMed
-
- Ali AT, Penny CB, Paiker JE, van Niekerk C, Smit A, Ferris WF, Crowther NJ. Alkaline phosphatase is involved in the control of adipogenesis in the murine preadipocyte cell line, 3T3-L1. Clinica Chimica Acta. 2005;354:101–109. - PubMed
-
- Asgari S, Zhang GM, Zareie R, Schmidt O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochemistry and Molecular Biology. 2003;33:1017–1024. - PubMed
-
- Asgari S. Venom proteins from polydnavirus-producing endoparasitoids : Their role in host-parasite interactions. Archives of Insect Biochemistry and Physiology. 2006;61:146–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

