Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design
- PMID: 20576091
- PMCID: PMC2908054
- DOI: 10.1186/1744-9081-6-34
Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design
Abstract
Background: Duration of efficacy and safety of lisdexamfetamine dimesylate (LDX) was assessed in adults (18-55 years) with attention-deficit/hyperactivity disorder (ADHD) using the simulated adult workplace environment.
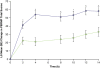
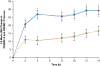
Methods: After open-label dose optimization (4-week) with LDX, 30-70 mg/d, subjects entered a 2-week randomized, double-blind, placebo-controlled crossover phase. Efficacy assessments included the Permanent Product Measure of Performance (PERMP) total score (attempted+correct) measured predose and from 2 to 14 hours postdose, averaged across postdose sessions (primary) and at each time point vs placebo (secondary), and ADHD Rating Scale IV (ADHD-RS-IV) with adult prompts at baseline and crossover visits. Safety assessments included treatment-emergent adverse events (TEAEs), vital signs, and electrocardiograms.
Results: Of 127 randomized subjects, 105 were in the intention-to-treat population and 103 completed the study. While receiving LDX vs placebo, adults had greater improvement (P < .0001) in average PERMP total scores as measured by difference in least squares (LS) mean (95% CI): 23.4 (15.6, 31.2). Absolute (P <or= .0017 for each time point) and change from predose (P < .001 for each time point) PERMP total scores were greater at all postdose time points from 2 to 14 h for adults while receiving LDX vs placebo. LDX demonstrated efficacy vs placebo (P < .0001) by the difference in LS mean (95% CI) for ADHD-RS-IV total scores: -11.5 (-14.2, -8.9). TEAEs (>or=10%) during dose optimization were decreased appetite, dry mouth, headache, and insomnia; no TEAEs >or=5% were reported during crossover phase for adults receiving LDX.
Conclusions: LDX significantly improved PERMP scores vs placebo and maintained improvement throughout the day from the first (2 hours) to last (14 hours) postdose time point vs placebo in adults with ADHD.
Trial registration: ClinicalTrials.gov Identifier: NCT00697515. Safety and Efficacy Workplace Environment Study of Lisdexamfetamine Dimesylate (LDX) in Adults With Attention-Deficit Hyperactivity Disorder (ADHD) http://www.clinicaltrials.gov/ct2/show/NCT00697515?term=NCT00697515&rank=1.
Figures


References
-
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. - DOI - PMC - PubMed
-
- Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, De GG, Haro JM, Karam EG, Lara C, Lepine JP, Ormel J, Posada-Villa J, Zaslavsky AM, Jin R. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402–409. doi: 10.1192/bjp.bp.106.034389. - DOI - PubMed

