Comparison of BCG, MPL and cationic liposome adjuvant systems in leishmanial antigen vaccine formulations against murine visceral leishmaniasis
- PMID: 20576102
- PMCID: PMC2904331
- DOI: 10.1186/1471-2180-10-181
Comparison of BCG, MPL and cationic liposome adjuvant systems in leishmanial antigen vaccine formulations against murine visceral leishmaniasis
Abstract
Background: The development of an effective vaccine against visceral leishmaniasis (VL) caused by Leishmania donovani is an essential aim for controlling the disease. Use of the right adjuvant is of fundamental importance in vaccine formulations for generation of effective cell-mediated immune response. Earlier we reported the protective efficacy of cationic liposome-associated L. donovani promastigote antigens (LAg) against experimental VL. The aim of the present study was to compare the effectiveness of two very promising adjuvants, Bacille Calmette-Guerin (BCG) and Monophosphoryl lipid A (MPL) plus trehalose dicorynomycolate (TDM) with cationic liposomes, in combination with LAg, to confer protection against murine VL.
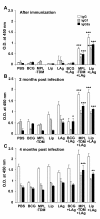
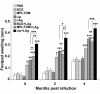
Results: All the three formulations afforded significant protection against L. donovani in both the visceral organs, liver and spleen. Although comparable level of protection was observed in BCG+LAg and MPL-TDM+LAg immunized mice, highest level of protection was exhibited by the liposomal LAg immunized group. Significant increase in anti-LAg IgG levels were detected in both MPL-TDM+LAg and liposomal LAg immunized animals with higher levels of IgG2a than IgG1. But BCG+LAg failed to induce any antibody response. As an index of cell-mediated immunity DTH responses were measured and significant response was observed in mice vaccinated with all the three different formulations. However, highest responses were observed with liposomal vaccine immunization. Comparative evaluation of IFN-gamma and IL-4 responses in immunized mice revealed that MPL-TDM+LAg group produced the highest level of IFN-gamma but lowest IL-4 level, while BCG+LAg demonstrated generation of suboptimum levels of both IFN-gamma and IL-4 response. Elicitation of moderate levels of prechallenge IFN-gamma along with optimum IL-4 corresponds with successful vaccination with liposomal LAg.
Conclusion: This comparative study reveals greater effectiveness of the liposomal vaccine for protection against progressive VL in BALB/c. Again, evaluation of the immune responses by vaccination emphasizes the need of stimulation of potent cellular immunity based on both Th1 and Th2 cell responses to confer protection against VL.
Figures
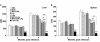
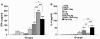
Similar articles
-
Vaccination with liposomal leishmanial antigens adjuvanted with monophosphoryl lipid-trehalose dicorynomycolate (MPL-TDM) confers long-term protection against visceral leishmaniasis through a human administrable route.Mol Pharm. 2012 Jan 1;9(1):59-70. doi: 10.1021/mp2002494. Epub 2011 Dec 14. Mol Pharm. 2012. PMID: 22133194
-
Evaluation of the immunoprophylactic potential of a killed vaccine candidate in combination with different adjuvants against murine visceral leishmaniasis.Parasitol Int. 2015 Feb;64(1):70-8. doi: 10.1016/j.parint.2014.10.003. Epub 2014 Oct 12. Parasitol Int. 2015. PMID: 25316605
-
Potentiating effects of MPL on DSPC bearing cationic liposomes promote recombinant GP63 vaccine efficacy: high immunogenicity and protection.PLoS Negl Trop Dis. 2011 Dec;5(12):e1429. doi: 10.1371/journal.pntd.0001429. Epub 2011 Dec 20. PLoS Negl Trop Dis. 2011. PMID: 22206029 Free PMC article.
-
Efficacy of vaccines based on chimeric or multiepitope antigens for protection against visceral leishmaniasis: A systematic review.PLoS Negl Trop Dis. 2024 Dec 31;18(12):e0012757. doi: 10.1371/journal.pntd.0012757. eCollection 2024 Dec. PLoS Negl Trop Dis. 2024. PMID: 39739955 Free PMC article.
-
Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review.Phytomedicine. 2019 Jul;60:152905. doi: 10.1016/j.phymed.2019.152905. Epub 2019 Mar 30. Phytomedicine. 2019. PMID: 31182297 Free PMC article. Review.
Cited by
-
Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus Syrian hamster model.Vet Res. 2011 Feb 23;42(1):39. doi: 10.1186/1297-9716-42-39. Vet Res. 2011. PMID: 21345200 Free PMC article. Review.
-
Sphingomyelin Liposomes Containing Soluble Leishmania major antigens Induced Strong Th2 Immune Response in BALB/c Mice.Iran J Basic Med Sci. 2013 Sep;16(9):965-72. Iran J Basic Med Sci. 2013. PMID: 24171074 Free PMC article.
-
Liposomal adjuvant development for leishmaniasis vaccines.Ther Adv Vaccines. 2017 Aug;5(4-5):85-101. doi: 10.1177/2051013617741578. Epub 2017 Nov 15. Ther Adv Vaccines. 2017. PMID: 29201374 Free PMC article. Review.
-
Vaccine Development Against Leishmania donovani.Front Immunol. 2012 May 15;3:99. doi: 10.3389/fimmu.2012.00099. eCollection 2012. Front Immunol. 2012. PMID: 22615707 Free PMC article.
-
Babassu aqueous extract (BAE) as an adjuvant for T helper (Th)1-dependent immune responses in mice of a Th2 immune response-prone strain.BMC Immunol. 2011 Jan 29;12:13. doi: 10.1186/1471-2172-12-13. BMC Immunol. 2011. PMID: 21276258 Free PMC article.
References
-
- von Meyenn F, Schaefer M, Weighardt H, Bauer S, Kirschning CJ, Wagner H, Sparwasser T. Toll-like receptor 9 contributes to recognition of Mycobacterium bovis Bacillus Calmette-Guerin by Flt3-ligand generated dendritic cells. Immunobiology. 2006;211:557–565. doi: 10.1016/j.imbio.2006.05.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

