Poly(I:C) induces intense expression of c-IAP2 and cooperates with an IAP inhibitor in induction of apoptosis in cancer cells
- PMID: 20576118
- PMCID: PMC2928000
- DOI: 10.1186/1471-2407-10-327
Poly(I:C) induces intense expression of c-IAP2 and cooperates with an IAP inhibitor in induction of apoptosis in cancer cells
Abstract
Background: There is increasing evidence that the toll-like receptor 3 (TLR3) is an interesting target for anti-cancer therapy. Unfortunately, most laboratory investigations about the impact of TLR3 stimulation on human malignant cells have been performed with very high concentrations--5 to 100 microg/ml--of the prototype TLR3 ligand, poly(I:C). In a previous study focused on a specific type of human carcinoma - nasopharyngeal carcinoma - we have shown that concentrations of poly(I:C) as low as 100 ng/ml are sufficient to induce apoptosis of malignant cells when combined to a pharmacological antagonist of the IAP family based on Smac mimicry.
Methods: This observation prompted us to investigate the contribution of the IAP family in cell response to poly(I:C) in a variety of human malignant cell types.
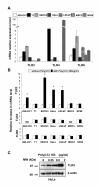
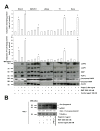
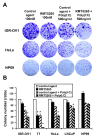
Results: We report a rapid, intense and selective increase in c-IAP2 protein expression observed under stimulation by poly(I:C)(500 ng/ml) in all types of human malignant cells. In most cell types, this change in protein expression is underlain by an increase in c-IAP2 transcripts and dependent on the TLR3/TRIF pathway. When poly(I:C) is combined to the IAP inhibitor RMT 5265, a cooperative effect in apoptosis induction and/or inhibition of clonogenic growth is obtained in a large fraction of carcinoma and melanoma cell lines.
Conclusions: Currently, IAP inhibitors like RMT 5265 and poly(I:C) are the subject of separate therapeutic trials. In light of our observations, combined use of both types of compounds should be considered for treatment of human malignancies including carcinomas and melanomas.
Figures





References
-
- Matsumoto M, Funami K, Oshiumi H, Seya T. Toll-like receptor 3: a link between toll-like receptor, interferon and viruses. Microbiol Immunol. 2004;48:147–154. - PubMed
-
- Laplanche A, Alzieu L, Delozier T, Berlie J, Veyret C, Fargeot P, Luboinski M, Lacour J. Polyadenylic-polyuridylic acid plus locoregional radiotherapy versus chemotherapy with CMF in operable breast cancer: a 14 year follow-up analysis of a randomized trial of the Federation Nationale des Centres de Lutte contre le Cancer (FNCLCC) Breast Cancer Res Treat. 2000;64:189–191. doi: 10.1023/A:1006498121628. - DOI - PubMed
-
- Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–4901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

