Biophysical characterization of DNA binding from single molecule force measurements
- PMID: 20576476
- PMCID: PMC2930095
- DOI: 10.1016/j.plrev.2010.06.001
Biophysical characterization of DNA binding from single molecule force measurements
Abstract
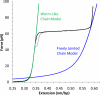
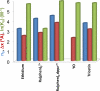
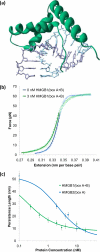
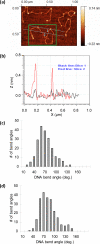
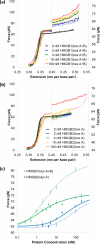
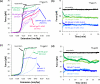
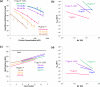
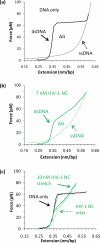
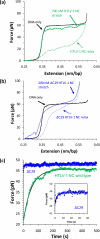
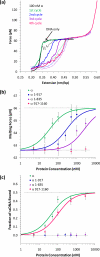
Single molecule force spectroscopy is a powerful method that uses the mechanical properties of DNA to explore DNA interactions. Here we describe how DNA stretching experiments quantitatively characterize the DNA binding of small molecules and proteins. Small molecules exhibit diverse DNA binding modes, including binding into the major and minor grooves and intercalation between base pairs of double-stranded DNA (dsDNA). Histones bind and package dsDNA, while other nuclear proteins such as high mobility group proteins bind to the backbone and bend dsDNA. Single-stranded DNA (ssDNA) binding proteins slide along dsDNA to locate and stabilize ssDNA during replication. Other proteins exhibit binding to both dsDNA and ssDNA. Nucleic acid chaperone proteins can switch rapidly between dsDNA and ssDNA binding modes, while DNA polymerases bind both forms of DNA with high affinity at distinct binding sites at the replication fork. Single molecule force measurements quantitatively characterize these DNA binding mechanisms, elucidating small molecule interactions and protein function.
(c) 2010 Elsevier B.V. All rights reserved.
Figures
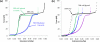


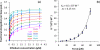

Comment in
-
Biophysics of DNA-ligand interactions resolved by force. Comment on "Biophysical characterization of DNA binding from single molecule force measurements" by K.R. Chaurasiya et al.Phys Life Rev. 2010 Sep;7(3):344-5; discussion 358-61. doi: 10.1016/j.plrev.2010.06.005. Epub 2010 Jun 25. Phys Life Rev. 2010. PMID: 20598655 No abstract available.
-
DNA binding by bacterial nucleoid proteins and the DNA overstretching transition. Comment on "Biophysical characterization of DNA binding from single molecule force measurements" by Mark C. Williams et al.Phys Life Rev. 2010 Sep;7(3):342-3; discussion 358-61. doi: 10.1016/j.plrev.2010.06.003. Epub 2010 Jun 25. Phys Life Rev. 2010. PMID: 20598658 No abstract available.
-
Microscopic implications of competing pictures of DNA overstretching. Comment on "Biophysical characterization of DNA binding from single molecule force measurements" by Kathy R. Chaurasiya, Thayaparan Paramanathan, Micah J. McCauley and Mark C. Williams.Phys Life Rev. 2010 Sep;7(3):348-9; discussion 358-61. doi: 10.1016/j.plrev.2010.06.009. Phys Life Rev. 2010. PMID: 20621570 No abstract available.
-
DNA-ligand binding and the force-extension experiments. Comment on "Biophysical characterization of DNA binding from single molecule force measurements" by Chaurasiya et al.Phys Life Rev. 2010 Sep;7(3):346-7; discussion 358-61. doi: 10.1016/j.plrev.2010.06.008. Phys Life Rev. 2010. PMID: 20621571 No abstract available.
-
Wonderful world of single biopolymer thermodynamics. Comment on "Biophysical characterization of DNA binding from single molecule force measurements" by K.R. Chaurasiya et al.Phys Life Rev. 2010 Sep;7(3):355-7; discussion 358-61. doi: 10.1016/j.plrev.2010.07.005. Epub 2010 Jul 22. Phys Life Rev. 2010. PMID: 20667796 No abstract available.
-
DNA overstretched state: S-DNA form or force-induced melting? Comment on "Biophysical characterization of DNA binding from single molecule force measurements" by Mark C. Williams et al.Phys Life Rev. 2010 Sep;7(3):350-2; discussion 358-61. doi: 10.1016/j.plrev.2010.07.001. Epub 2010 Jul 22. Phys Life Rev. 2010. PMID: 20673652 No abstract available.
-
Single molecule force measurements: insights from molecular simulations. Comment on "Biophysical characterization of DNA binding from single molecule force measurements" by Kathy R. Chaurasiya et al.Phys Life Rev. 2010 Sep;7(3):353-4; discussion 358-61. doi: 10.1016/j.plrev.2010.07.004. Epub 2010 Jul 22. Phys Life Rev. 2010. PMID: 20673653 No abstract available.
References
-
- Kasas S, Thomson NH, Smith BL, Hansma HG, Zhu XS, Guthold M, Bustamante C, Kool ET, Kashlev M, Hansma PK. Escherichia coli RNA polymerase activity observed using atomic force microscopy. Biochemistry. 1997;36:461–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

