Bilateral cochlear implantation in the ferret: a novel animal model for behavioral studies
- PMID: 20576507
- PMCID: PMC2938482
- DOI: 10.1016/j.jneumeth.2010.05.014
Bilateral cochlear implantation in the ferret: a novel animal model for behavioral studies
Abstract
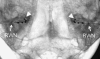
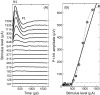
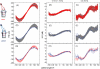
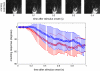
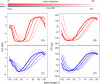
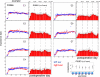
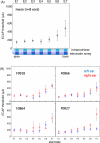
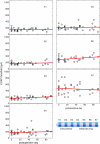
Bilateral cochlear implantation has recently been introduced with the aim of improving both speech perception in background noise and sound localization. Although evidence suggests that binaural perception is possible with two cochlear implants, results in humans are variable. To explore potential contributing factors to these variable outcomes, we have developed a behavioral animal model of bilateral cochlear implantation in a novel species, the ferret. Although ferrets are ideally suited to psychophysical and physiological assessments of binaural hearing, cochlear implantation has not been previously described in this species. This paper describes the techniques of deafening with aminoglycoside administration, surgical implantation of an intracochlear array and chronic intracochlear electrical stimulation with monitoring for electrode integrity and efficacy of stimulation. Experiments have been presented elsewhere to show that the model can be used to study behavioral and electrophysiological measures of binaural hearing in chronically implanted animals. This paper demonstrates that cochlear implantation and chronic intracochlear electrical stimulation are both safe and effective in ferrets, opening up the possibility of using this model to study potential protective effects of bilateral cochlear implantation on the developing central auditory pathway. Since ferrets can be used to assess psychophysical and physiological aspects of hearing along with the structure of the auditory pathway in the same animals, we anticipate that this model will help develop novel neuroprosthetic therapies for use in humans.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Benefits of bilateral electrical stimulation with the nucleus cochlear implant in adults: 6-month postoperative results.Otol Neurotol. 2004 Nov;25(6):958-68. doi: 10.1097/00129492-200411000-00016. Otol Neurotol. 2004. PMID: 15547426 Clinical Trial.
-
Cortical Representation of Interaural Time Difference Is Impaired by Deafness in Development: Evidence from Children with Early Long-term Access to Sound through Bilateral Cochlear Implants Provided Simultaneously.J Neurosci. 2017 Mar 1;37(9):2349-2361. doi: 10.1523/JNEUROSCI.2538-16.2017. Epub 2017 Jan 25. J Neurosci. 2017. PMID: 28123078 Free PMC article.
-
Using evoked potentials to match interaural electrode pairs with bilateral cochlear implants.J Assoc Res Otolaryngol. 2007 Mar;8(1):134-51. doi: 10.1007/s10162-006-0069-0. Epub 2007 Jan 17. J Assoc Res Otolaryngol. 2007. PMID: 17225976 Free PMC article.
-
What is the optimal timing for bilateral cochlear implantation in children?Cochlear Implants Int. 2011 Aug;12 Suppl 2:S8-14. doi: 10.1179/146701011X13074645127199. Cochlear Implants Int. 2011. PMID: 21917210 Review.
-
Benefits of bilateral cochlear implantation: a review.Curr Opin Otolaryngol Head Neck Surg. 2007 Oct;15(5):315-8. doi: 10.1097/MOO.0b013e3282ef3d3e. Curr Opin Otolaryngol Head Neck Surg. 2007. PMID: 17823546 Review.
Cited by
-
Can training extend current guidelines for cochlear implant candidacy?Neural Regen Res. 2015 May;10(5):718-20. doi: 10.4103/1673-5374.156964. Neural Regen Res. 2015. PMID: 26109944 Free PMC article. No abstract available.
-
Crossmodal integration improves sensory detection thresholds in the ferret.PLoS One. 2015 May 13;10(5):e0124952. doi: 10.1371/journal.pone.0124952. eCollection 2015. PLoS One. 2015. PMID: 25970327 Free PMC article.
-
Behavioural sensitivity to binaural spatial cues in ferrets: evidence for plasticity in the duplex theory of sound localization.Eur J Neurosci. 2014 Jan;39(2):197-206. doi: 10.1111/ejn.12402. Epub 2013 Oct 28. Eur J Neurosci. 2014. PMID: 24256073 Free PMC article.
-
Auditory cortical plasticity in cochlear implant users.Curr Opin Neurobiol. 2020 Feb;60:108-114. doi: 10.1016/j.conb.2019.11.003. Epub 2019 Dec 18. Curr Opin Neurobiol. 2020. PMID: 31864104 Free PMC article. Review.
-
A physiological and behavioral system for hearing restoration with cochlear implants.J Neurophysiol. 2016 Aug 1;116(2):844-58. doi: 10.1152/jn.00048.2016. Epub 2016 Jun 8. J Neurophysiol. 2016. PMID: 27281743 Free PMC article.
References
-
- Beitel R.E., Snyder R.L., Schreiner C.E., Raggio M.W., Leake P.A. Electrical cochlear stimulation in the deaf cat: comparisons between psychophysical and central auditory neuronal thresholds. J Neurophysiol. 2000;83:2145–2162. - PubMed
-
- Bizley J.K., Nodal F.R., Nelken I., King A.J. Functional organization of ferret auditory cortex. Cereb Cortex. 2005;15:1637–1653. - PubMed
-
- Brown C.J., Abbas P.J., Gantz B. Electrically evoked whole-nerve action potentials: data from human cochlear implant users. J Acoust Soc Am. 1990;88:1385–1391. - PubMed
-
- Dahmen J.C., King A.J. Learning to hear: plasticity of auditory cortical processing. Curr Opin Neurobiol. 2007;17:456–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

