Heparan sulfate domain organization and sulfation modulate FGF-induced cell signaling
- PMID: 20576609
- PMCID: PMC2930683
- DOI: 10.1074/jbc.M109.093542
Heparan sulfate domain organization and sulfation modulate FGF-induced cell signaling
Abstract
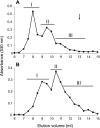
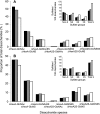
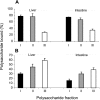
Heparan sulfates (HSs) modulate various developmental and homeostatic processes by binding to protein ligands. We have evaluated the structural characteristics of porcine HS in cellular signaling induced by basic fibroblast growth factor (FGF2), using CHO745 cells devoid of endogenous glycosaminoglycans as target. Markedly enhanced stimulation of cell signaling, measured as phosphorylation of ERK1/2 and protein kinase B, was only observed with the shortest HS chains isolated from liver, whereas the longer chains from either liver or intestine essentially prolonged duration of signals induced by FGF2 in the absence of polysaccharide. Structural analysis showed that contiguous sulfated domains were most abundant in the shortest HS chains and were more heavily sulfated in HS from liver than in HS from intestine. Moreover, the shortest chains from either source entered into ternary complexes with FGF2 and FGF receptor-1c more efficiently than the corresponding longer chains. In addition to authentic HSs, decasaccharide libraries generated by chemo-enzymatic modification of heparin were probed for effect on FGF2 signaling. Only the most highly sulfated decamers, previously found most efficient in ternary complex formation (Jastrebova, N., Vanwildemeersch, M., Rapraeger, A. C., Giménez-Gallego, G., Lindahl, U., and Spillmann, D. (2006) J. Biol. Chem. 281, 26884-26892), promoted FGF2 cellular signaling as efficiently as short HS chains from liver. Together these results suggest that the effects of HS on FGF2 signaling are determined by both the structure of the highly sulfated domains and by the organization/availability of such domains within the HS chain. These findings underpin the need for regulation of HS biosynthesis in relation to control of growth factor-induced signaling pathways.
Figures





Similar articles
-
Specific inhibition of FGF-2 signaling with 2-O-sulfated octasaccharides of heparan sulfate.Glycobiology. 2009 Jun;19(6):644-54. doi: 10.1093/glycob/cwp031. Epub 2009 Mar 2. Glycobiology. 2009. PMID: 19254961
-
Highly sulfated nonreducing end-derived heparan sulfate domains bind fibroblast growth factor-2 with high affinity and are enriched in biologically active fractions.J Biol Chem. 2011 Jun 3;286(22):19311-9. doi: 10.1074/jbc.M110.204693. Epub 2011 Apr 6. J Biol Chem. 2011. PMID: 21471211 Free PMC article.
-
Minimum FGF2 binding structural requirements of heparin and heparan sulfate oligosaccharides as determined by NMR spectroscopy.Biochemistry. 2008 Dec 30;47(52):13862-9. doi: 10.1021/bi801007p. Biochemistry. 2008. PMID: 19117094
-
Heparin-derived heparan sulfate mimics to modulate heparan sulfate-protein interaction in inflammation and cancer.Matrix Biol. 2010 Jul;29(6):442-52. doi: 10.1016/j.matbio.2010.04.003. Epub 2010 Apr 21. Matrix Biol. 2010. PMID: 20416374 Free PMC article. Review.
-
Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: mechanisms and mysteries.Connect Tissue Res. 2015;56(4):272-80. doi: 10.3109/03008207.2015.1045066. Connect Tissue Res. 2015. PMID: 26076122 Free PMC article. Review.
Cited by
-
Host Cell Receptors Implicated in the Cellular Tropism of BVDV.Viruses. 2022 Oct 20;14(10):2302. doi: 10.3390/v14102302. Viruses. 2022. PMID: 36298858 Free PMC article. Review.
-
Demystifying heparan sulfate-protein interactions.Annu Rev Biochem. 2014;83:129-57. doi: 10.1146/annurev-biochem-060713-035314. Epub 2014 Mar 6. Annu Rev Biochem. 2014. PMID: 24606135 Free PMC article. Review.
-
Extracellular distribution of diffusible growth factors controlled by heparan sulfate proteoglycans during mammalian embryogenesis.Philos Trans R Soc Lond B Biol Sci. 2014 Dec 5;369(1657):20130545. doi: 10.1098/rstb.2013.0545. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25349453 Free PMC article. Review.
-
HSulf-1 deficiency dictates a metabolic reprograming of glycolysis and TCA cycle in ovarian cancer.Oncotarget. 2015 Oct 20;6(32):33705-19. doi: 10.18632/oncotarget.5605. Oncotarget. 2015. PMID: 26378042 Free PMC article.
-
Xylosyltransferase I mediates the synthesis of proteoglycans with long glycosaminoglycan chains and controls chondrocyte hypertrophy and collagen fibers organization of in the growth plate.Cell Death Dis. 2023 Jun 9;14(6):355. doi: 10.1038/s41419-023-05875-0. Cell Death Dis. 2023. PMID: 37296099 Free PMC article.
References
-
- Bernfield M., Götte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. (1999) Annu. Rev. Biochem. 68, 729–777 - PubMed
-
- Iozzo R. V. (2005) Nat. Rev. Mol. Cell Biol. 6, 646–656 - PubMed
-
- Maccarana M., Sakura Y., Tawada A., Yoshida K., Lindahl U. (1996) J. Biol. Chem. 271, 17804–17810 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

