Chronic hyperoxia alters the early and late phases of the hypoxic ventilatory response in neonatal rats
- PMID: 20576840
- PMCID: PMC2944635
- DOI: 10.1152/japplphysiol.00510.2010
Chronic hyperoxia alters the early and late phases of the hypoxic ventilatory response in neonatal rats
Abstract
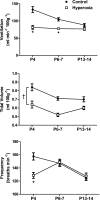
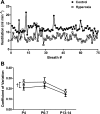
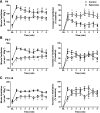
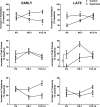
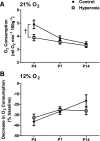
Chronic hyperoxia during the first 1-4 postnatal weeks attenuates the hypoxic ventilatory response (HVR) subsequently measured in adult rats. Rather than focusing on this long-lasting plasticity, the present study considered the influence of hyperoxia on respiratory control during the neonatal period. Sprague-Dawley rats were born and raised in 60% O2 until studied at postnatal ages (P) of 4, 6-7, or 13-14 days. Ventilation and metabolism were measured in normoxia (21% O2) and acute hypoxia (12% O2) using head-body plethysmography and respirometry, respectively. Compared with age-matched rats raised in room air, the major findings were 1) diminished pulmonary ventilation and metabolic O2 consumption in normoxia at P4 and P6-7; 2) decreased breathing stability during normoxia; 3) attenuation of the early phase of the HVR at P6-7 and P13-14; and 4) a sustained increase in ventilation during hypoxia (vs. the normal biphasic HVR) at all ages studied. Attenuation of the early HVR likely reflects progressive impairment of peripheral arterial chemoreceptors while expression of a sustained HVR in neonates before P7 suggests that hyperoxia also induces plasticity within the central nervous system. Together, these results suggest a complex interaction between inhibitory and excitatory effects of hyperoxia on the developing respiratory control system.
Figures
References
-
- Bavis RW, Mitchell GS. Long-term effects of the perinatal environment on respiratory control. J Appl Physiol 104: 1220–1229, 2008 - PubMed
-
- Bavis RW, Olson EB, Jr, Mitchell GS. Critical developmental period for hyperoxia-induced blunting of hypoxic phrenic responses in rats. J Appl Physiol 92: 1013–1018, 2002 - PubMed
-
- Bavis RW, Russell KER, Simons JC, Otis JP. Hypoxic ventilatory responses in rats after hypercapnic hyperoxia and intermittent hyperoxia. Respir Physiol Neurobiol 155: 193–202, 2007 - PubMed
-
- Bavis RW, Olson EB, Jr, Vidruk EH, Bisgard GE, Mitchell GS. Level and duration of developmental hyperoxia influence impairment of hypoxic phrenic responses in rats. J Appl Physiol 95: 1550–1559, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

