Cancellous bone adaptation to tibial compression is not sex dependent in growing mice
- PMID: 20576844
- PMCID: PMC3137235
- DOI: 10.1152/japplphysiol.00210.2010
Cancellous bone adaptation to tibial compression is not sex dependent in growing mice
Abstract
Mechanical loading can be used to increase bone mass and thus attenuate pathological bone loss. Because the skeleton's adaptive response to loading is most robust before adulthood, elucidating sex-specific responses during growth may help maximize peak bone mass. This study investigated the effect of sex on the response to controlled, in vivo mechanical loading in growing mice. Ten-week-old male and female C57Bl/6 mice underwent noninvasive compression of the left tibia. Peak loads of -11.5 N were applied, corresponding to +1,200 microepsilon at the tibial midshaft in both sexes. Cancellous bone mass, architecture, and dynamic formation in the proximal metaphysis were compared between loaded and control limbs via micro-computed tomography and histomorphometry. The strain environment of the proximal metaphysis during loading was characterized using finite element analysis. Both sexes responded to tibial compression through increased bone mass and altered architecture. Cancellous bone mass and tissue density were enhanced in loaded limbs relative to control limbs in both sexes through trabecular thickening and reduced separation. Changes in mass were due to increased cellular activity in loaded limbs compared with control limbs. Adaptation to loading increased the proportion of load transferred by the cancellous bone in the proximal metaphysis. For all cancellous measures, the response to tibial compression did not differ between male and female mice. When similar strains are engendered in males and females, the adaptive response in cancellous bone to mechanical loading does not depend on sex.
Figures
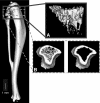

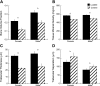
Similar articles
-
Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging.Bone. 2011 Sep;49(3):439-46. doi: 10.1016/j.bone.2011.05.017. Epub 2011 May 27. Bone. 2011. PMID: 21642027 Free PMC article.
-
Load-induced changes in bone stiffness and cancellous and cortical bone mass following tibial compression diminish with age in female mice.J Exp Biol. 2014 May 15;217(Pt 10):1775-83. doi: 10.1242/jeb.085522. Epub 2014 Feb 27. J Exp Biol. 2014. PMID: 24577445 Free PMC article.
-
Adaptive changes in micromechanical environments of cancellous and cortical bone in response to in vivo loading and disuse.J Biomech. 2019 May 24;89:85-94. doi: 10.1016/j.jbiomech.2019.04.021. Epub 2019 Apr 19. J Biomech. 2019. PMID: 31047696
-
Characterization of cancellous and cortical bone strain in the in vivo mouse tibial loading model using microCT-based finite element analysis.Bone. 2014 Sep;66:131-9. doi: 10.1016/j.bone.2014.05.019. Epub 2014 Jun 9. Bone. 2014. PMID: 24925445
-
Methodological aspects of in vivo axial loading in rodents: a systematic review.J Musculoskelet Neuronal Interact. 2023 Jun 1;23(2):236-262. J Musculoskelet Neuronal Interact. 2023. PMID: 37259664 Free PMC article.
Cited by
-
Connexin hemichannels with prostaglandin release in anabolic function of bone to mechanical loading.Elife. 2022 Feb 8;11:e74365. doi: 10.7554/eLife.74365. Elife. 2022. PMID: 35132953 Free PMC article.
-
In vivo axial loading of the mouse tibia.Methods Mol Biol. 2015;1226:99-115. doi: 10.1007/978-1-4939-1619-1_9. Methods Mol Biol. 2015. PMID: 25331046 Free PMC article.
-
Effects of Loading Duration and Short Rest Insertion on Cancellous and Cortical Bone Adaptation in the Mouse Tibia.PLoS One. 2017 Jan 11;12(1):e0169519. doi: 10.1371/journal.pone.0169519. eCollection 2017. PLoS One. 2017. PMID: 28076363 Free PMC article.
-
Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging.Bone. 2011 Sep;49(3):439-46. doi: 10.1016/j.bone.2011.05.017. Epub 2011 May 27. Bone. 2011. PMID: 21642027 Free PMC article.
-
Old Mice Have Less Transcriptional Activation But Similar Periosteal Cell Proliferation Compared to Young-Adult Mice in Response to in vivo Mechanical Loading.J Bone Miner Res. 2020 Sep;35(9):1751-1764. doi: 10.1002/jbmr.4031. Epub 2020 Jun 1. J Bone Miner Res. 2020. PMID: 32311160 Free PMC article.
References
-
- Ballard TL, Specker BL, Binkley TL, Vukovich MD. Effect of protein supplementation during a 6-mo strength and conditioning program on areal and volumetric bone parameters. Bone 38: 898–904, 2006 - PubMed
-
- Biewener AA. Safety factors in bone strength. Calcif Tissue Int 53, Suppl 1: S68–S74, 1993 - PubMed
-
- Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women: interaction of mechanical, hormonal and dietary factors. Sports Med 35: 779–830, 2005 - PubMed
-
- Bourne BC, van der Meulen MC. Finite element models predict cancellous apparent modulus when tissue modulus is scaled from specimen CT-attenuation. J Biomech 37: 613–621, 2004 - PubMed
-
- Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res 14: 2159–2166, 1999 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

