Innate immune processes are sufficient for driving silicosis in mice
- PMID: 20576854
- PMCID: PMC2924603
- DOI: 10.1189/jlb.0210108
Innate immune processes are sufficient for driving silicosis in mice
Abstract
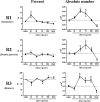
The lung is constantly exposed to potentially pathogenic particles and microorganisms. It has become evident recently that not only innate but also adaptive immune responses to particulates, such as SiO(2) entering the respiratory tract, are complex and dynamic events. Although the cellular mechanisms and anatomical consequences involved in the development of silicosis have been studied extensively, they still remain poorly understood. Based on their capacity for immune regulation, lymphocytes may play a key role in the respiratory response to environmental challenge by SiO(2). The objective of this study was to characterize the impact of SiO(2) exposure on respiratory immune processes, with particular emphasis on evaluating the importance of lymphocytes in the murine silicosis model. Therefore, lymphopenic mice, including NK-deficient, Rag1(-/-), or a combination (Rag1(-/-) NK-depleted), were used and demonstrated that SiO(2)-induced fibrosis and inflammation can occur independently of T, B, NK T, and NK cells. Studies in Rag1(-/-) mice suggest further that lymphocytes may participate in the regulation of SiO(2)-induced inflammation through modulation of the Nalp3 inflammasome. This observation may have clinical relevance in the treatment of inflammatory and fibrotic lung diseases that are refractory or respond suboptimally to current therapeutics.
Figures
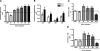



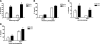
Similar articles
-
IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis.J Immunol. 2010 Jun 1;184(11):6367-77. doi: 10.4049/jimmunol.0900459. Epub 2010 Apr 26. J Immunol. 2010. PMID: 20421647
-
Mechanics-activated fibroblasts promote pulmonary group 2 innate lymphoid cell plasticity propelling silicosis progression.Nat Commun. 2024 Nov 12;15(1):9770. doi: 10.1038/s41467-024-54174-5. Nat Commun. 2024. PMID: 39532893 Free PMC article.
-
Interferon-gamma production by specific lung lymphocyte phenotypes in silicosis in mice.Am J Respir Cell Mol Biol. 2000 Apr;22(4):491-501. doi: 10.1165/ajrcmb.22.4.3599. Am J Respir Cell Mol Biol. 2000. PMID: 10745030
-
New developments in the understanding of immunology in silicosis.Curr Opin Allergy Clin Immunol. 2007 Apr;7(2):168-73. doi: 10.1097/ACI.0b013e32802bf8a5. Curr Opin Allergy Clin Immunol. 2007. PMID: 17351471 Review.
-
Lymphocytes, lymphokines, and silicosis.J Environ Pathol Toxicol Oncol. 2001;20 Suppl 1:53-65. J Environ Pathol Toxicol Oncol. 2001. PMID: 11570674 Review.
Cited by
-
Rapid Induction of Pulmonary Inflammation, Autoimmune Gene Expression, and Ectopic Lymphoid Neogenesis Following Acute Silica Exposure in Lupus-Prone Mice.Front Immunol. 2021 Feb 23;12:635138. doi: 10.3389/fimmu.2021.635138. eCollection 2021. Front Immunol. 2021. PMID: 33732257 Free PMC article.
-
Immunophenotyping of Acute Inflammatory Exacerbations of Lung Injury Driven by Mutant Surfactant Protein-C: A Role for Inflammatory Eosinophils.Front Pharmacol. 2022 Apr 27;13:875887. doi: 10.3389/fphar.2022.875887. eCollection 2022. Front Pharmacol. 2022. PMID: 35571100 Free PMC article.
-
Combined Action of Human Commensal Bacteria and Amorphous Silica Nanoparticles on the Viability and Immune Responses of Dendritic Cells.Clin Vaccine Immunol. 2017 Oct 5;24(10):e00178-17. doi: 10.1128/CVI.00178-17. Print 2017 Oct. Clin Vaccine Immunol. 2017. PMID: 28835358 Free PMC article.
-
Co-localization of iron binding on silica with p62/sequestosome1 (SQSTM1) in lung granulomas of mice with acute silicosis.J Clin Biochem Nutr. 2015 Jan;56(1):74-83. doi: 10.3164/jcbn.14-44. Epub 2014 Nov 28. J Clin Biochem Nutr. 2015. PMID: 25834305 Free PMC article.
-
Differences in gene expression and cytokine production by crystalline vs. amorphous silica in human lung epithelial cells.Part Fibre Toxicol. 2012 Feb 2;9(1):6. doi: 10.1186/1743-8977-9-6. Part Fibre Toxicol. 2012. PMID: 22300531 Free PMC article.
References
-
- Parks C G, Cooper G S, Nylander-French L A, Sanderson W T, Dement J M, Cohen P L, Dooley M A, Treadwell E L, St Clair E W, Gilkeson G S, Hoppin J A, Savitz D A. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case-control study in the southeastern United States. Arthritis Rheum. 2002;46:1840–1850. - PubMed
-
- Ding M, Chen F, Shi X, Yucesoy B, Mossman B, Vallyathan V. Diseases caused by silica: mechanisms of injury and disease development. Int Immunopharmacol. 2002;2:173–182. - PubMed
-
- Arras M, Louahed J, Simoen V, Barbarin V, Misson P, van den Brule S, Delos M, Knoops L, Renauld J C, Lison D, Huaux F. B lymphocytes are critical for lung fibrosis control and prostaglandin E2 regulation in IL-9 transgenic mice. Am J Respir Cell Mol Biol. 2006;34:573–580. - PubMed
-
- Hubbard A K. Role for T lymphocytes in silica-induced pulmonary inflammation. Lab Invest. 1989;61:46–52. - PubMed
-
- Davis G S, Holmes C E, Pfeiffer L M, Hemenway D R. Lymphocytes, lymphokines, and silicosis. J Environ Pathol Toxicol Oncol. 2001;20 (Suppl. 1):53–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

