Inactivation of hepatocyte nuclear factor-4{alpha} mediates alcohol-induced downregulation of intestinal tight junction proteins
- PMID: 20576917
- PMCID: PMC2950677
- DOI: 10.1152/ajpgi.00515.2009
Inactivation of hepatocyte nuclear factor-4{alpha} mediates alcohol-induced downregulation of intestinal tight junction proteins
Abstract
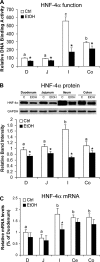
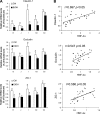
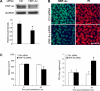
Chronic alcohol exposure has been shown to increase the gut permeability in the distal intestine, in part, through induction of zinc deficiency. The present study evaluated the molecular mechanisms whereby zinc deficiency mediates alcohol-induced intestinal barrier dysfunction. Examination of zinc finger transcription factors in the gastrointestinal tract of mice revealed a prominent distribution of hepatocyte nuclear factor-4alpha (HNF-4alpha). HNF-4alpha exclusively localizes in the epithelial nuclei and exhibited an increased abundance in mRNA and protein levels in the distal intestine. Chronic alcohol exposure to mice repressed the HNF-4alpha gene expression in the ileum and reduced the protein level and DNA binding activity of HNF-4alpha in all of the intestinal segments with the most remarkable changes in the ileum. Chronic alcohol exposure also decreased the mRNA levels of tight junction proteins, particularly in the ileum. Caco-2 cell culture studies were conducted to determine the role of HNF-4alpha in regulation of the epithelial tight junction and barrier function. Knockdown of HNF-4alpha in Caco-2 cells decreased the mRNA and protein levels of tight junction proteins in association with disruption of the epithelial barrier. Alcohol treatment inactivated HNF-4alpha, which was prevented by N-acetyl-cysteine or zinc. The link between zinc and HNF-4alpha function was confirmed by zinc deprivation, which inhibited HNF-4alpha DNA binding activity. These results indicate that inactivation of HNF-4alpha due to oxidative stress and zinc deficiency is likely a novel mechanism contributing to the deleterious effects of alcohol on the tight junctions and the intestinal barrier function.
Figures


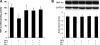
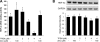
Similar articles
-
Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium.Mol Cell Biol. 2009 Dec;29(23):6294-308. doi: 10.1128/MCB.00939-09. Epub 2009 Oct 5. Mol Cell Biol. 2009. PMID: 19805521 Free PMC article.
-
Hepatocyte nuclear factor 4α regulates the expression of intestinal epithelial Na+/H+ exchanger isoform 3.Am J Physiol Gastrointest Liver Physiol. 2018 Jan 1;314(1):G14-G21. doi: 10.1152/ajpgi.00225.2017. Epub 2017 Sep 7. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 28882825 Free PMC article.
-
Hepatocyte nuclear factor (HNF)-4alpha triggers formation of functional tight junctions and establishment of polarized epithelial morphology in F9 embryonal carcinoma cells.Exp Cell Res. 2003 Jun 10;286(2):288-97. doi: 10.1016/s0014-4827(03)00116-2. Exp Cell Res. 2003. PMID: 12749857
-
Zinc and alcoholic liver disease.Dig Dis. 2010;28(6):745-50. doi: 10.1159/000324282. Epub 2011 Apr 27. Dig Dis. 2010. PMID: 21525759 Free PMC article. Review.
-
Systematic-Narrative Hybrid Literature Review: Crosstalk between Gastrointestinal Renin-Angiotensin and Dopaminergic Systems in the Regulation of Intestinal Permeability by Tight Junctions.Int J Mol Sci. 2024 May 20;25(10):5566. doi: 10.3390/ijms25105566. Int J Mol Sci. 2024. PMID: 38791603 Free PMC article. Review.
Cited by
-
Translational Approaches with Antioxidant Phytochemicals against Alcohol-Mediated Oxidative Stress, Gut Dysbiosis, Intestinal Barrier Dysfunction, and Fatty Liver Disease.Antioxidants (Basel). 2021 Mar 4;10(3):384. doi: 10.3390/antiox10030384. Antioxidants (Basel). 2021. PMID: 33806556 Free PMC article. Review.
-
Alcoholic Liver Disease: Update on the Role of Dietary Fat.Biomolecules. 2016 Jan 6;6(1):1. doi: 10.3390/biom6010001. Biomolecules. 2016. PMID: 26751488 Free PMC article. Review.
-
Lactobacillus johnsonii BS15 improves intestinal environment against fluoride-induced memory impairment in mice-a study based on the gut-brain axis hypothesis.PeerJ. 2020 Oct 7;8:e10125. doi: 10.7717/peerj.10125. eCollection 2020. PeerJ. 2020. PMID: 33083147 Free PMC article.
-
Targeting the gut barrier for the treatment of alcoholic liver disease.Liver Res. 2017 Dec;1(4):197-207. doi: 10.1016/j.livres.2017.12.004. Liver Res. 2017. PMID: 30034913 Free PMC article.
-
The Influence of Alcohol Consumption on Intestinal Nutrient Absorption: A Comprehensive Review.Nutrients. 2023 Mar 24;15(7):1571. doi: 10.3390/nu15071571. Nutrients. 2023. PMID: 37049411 Free PMC article. Review.
References
-
- Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction, and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther 291: 1075–1085, 1999 - PubMed
-
- Banan A, Farhadi A, Fields JZ, Mutlu E, Zhang L, Keshavarzian A. Evidence that nuclear factor-kappa B activation is critical in oxidant-induced disruption of the microtubule cytoskeleton, and barrier integrity and that its inactivation is essential in epidermal growth factor-mediated protection of the monolayers of intestinal epithelia. J Pharmacol Exp Ther 306: 13–28, 2003 - PubMed
-
- Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther 294: 997–1008, 2000 - PubMed
-
- Banan A, Zhang LJ, Farhadi A, Fields JZ, Shaikh M, Keshavarzian A. PKCβ1 isoform activation is required for EGF-induced NF-κB inactivation, and IκBα stabilization and protection of F-actin assembly and barrier function in enterocyte monolayers. Am J Physiol Cell Physiol 286: C723–C738, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

