T-tubule remodeling during transition from hypertrophy to heart failure
- PMID: 20576937
- PMCID: PMC2927862
- DOI: 10.1161/CIRCRESAHA.109.212324
T-tubule remodeling during transition from hypertrophy to heart failure
Abstract
Rationale: The transverse tubule (T-tubule) system is the ultrastructural substrate for excitation-contraction coupling in ventricular myocytes; T-tubule disorganization and loss are linked to decreased contractility in end stage heart failure (HF).
Objective: We sought to examine (1) whether pathological T-tubule remodeling occurs early in compensated hypertrophy and, if so, how it evolves during the transition from hypertrophy to HF; and (2) the role of junctophilin-2 in T-tubule remodeling.
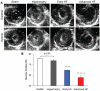
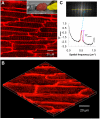
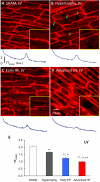
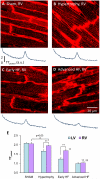
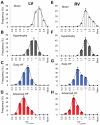
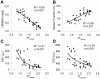
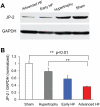
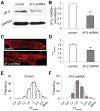
Methods and results: We investigated T-tubule remodeling in relation to ventricular function during HF progression using state-of-the-art confocal imaging of T-tubules in intact hearts, using a thoracic aortic banding rat HF model. We developed a quantitative T-tubule power (TT(power)) index to represent the integrity of T-tubule structure. We found that discrete local loss and global reorganization of the T-tubule system (leftward shift of TT(power) histogram) started early in compensated hypertrophy in left ventricular (LV) myocytes, before LV dysfunction, as detected by echocardiography. With progression from compensated hypertrophy to early and late HF, T-tubule remodeling spread from the LV to the right ventricle, and TT(power) histograms of both ventricles gradually shifted leftward. The mean LV TT(power) showed a strong correlation with ejection fraction and heart weight to body weight ratio. Over the progression to HF, we observed a gradual reduction in the expression of a junctophilin protein (JP-2) implicated in the formation of T-tubule/sarcoplasmic reticulum junctions. Furthermore, we found that JP-2 knockdown by gene silencing reduced T-tubule structure integrity in cultured adult ventricular myocytes.
Conclusions: T-tubule remodeling in response to thoracic aortic banding stress begins before echocardiographically detectable LV dysfunction and progresses over the development of overt structural heart disease. LV T-tubule remodeling is closely associated with the severity of cardiac hypertrophy and predicts LV function. Thus, T-tubule remodeling may constitute a key mechanism underlying the transition from compensated hypertrophy to HF.
Figures
Comment in
-
Cardiomyocyte transverse tubule loss leads the way to heart failure.Future Cardiol. 2011 Jan;7(1):39-42. doi: 10.2217/fca.10.113. Future Cardiol. 2011. PMID: 21174508
References
-
- Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. - PubMed
-
- Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ.Res. 2003;92:1182–1192. - PubMed
-
- Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ.Res. 2003;93:487–490. - PubMed
-
- Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ.Res. 2003;92:350–358. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

