Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells
- PMID: 20577048
- PMCID: PMC2898590
- DOI: 10.1172/JCI41203
Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells
Abstract
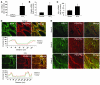
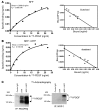
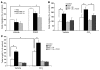
PDGF-dependent hepatic stellate cell (HSC) recruitment is an essential step in liver fibrosis and the sinusoidal vascular changes that accompany this process. However, the mechanisms that regulate PDGF signaling remain incompletely defined. Here, we found that in two rat models of liver fibrosis, the axonal guidance molecule neuropilin-1 (NRP-1) was upregulated in activated HSCs, which exhibit the highly motile myofibroblast phenotype. Additionally, NRP-1 colocalized with PDGF-receptor beta (PDGFRbeta) in HSCs both in the injury models and in human and rat HSC cell lines. In human HSCs, siRNA-mediated knockdown of NRP-1 attenuated PDGF-induced chemotaxis, while NRP-1 overexpression increased cell motility and TGF-beta-dependent collagen production. Similarly, mouse HSCs genetically modified to lack NRP-1 displayed reduced motility in response to PDGF treatment. Immunoprecipitation and biochemical binding studies revealed that NRP-1 increased PDGF binding affinity for PDGFRbeta-expressing cells and promoted downstream signaling. An NRP-1 neutralizing Ab ameliorated recruitment of HSCs, blocked liver fibrosis in a rat model of liver injury, and also attenuated VEGF responses in cultured liver endothelial cells. In addition, NRP-1 overexpression was observed in human specimens of liver cirrhosis caused by both hepatitis C and steatohepatitis. These studies reveal a role for NRP-1 as a modulator of multiple growth factor targets that regulate liver fibrosis and the vascular changes that accompany it and may have broad implications for liver cirrhosis and myofibroblast biology in a variety of other organ systems and disease conditions.
Figures






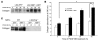

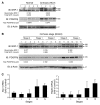
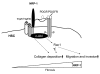
Comment in
-
Neuropilin and liver fibrosis: hitting three birds with one stone?Hepatology. 2011 Sep 2;54(3):1091-3. doi: 10.1002/hep.24484. Hepatology. 2011. PMID: 22179987 No abstract available.
Similar articles
-
Glycosylation-dependent galectin-1/neuropilin-1 interactions promote liver fibrosis through activation of TGF-β- and PDGF-like signals in hepatic stellate cells.Sci Rep. 2017 Sep 8;7(1):11006. doi: 10.1038/s41598-017-11212-1. Sci Rep. 2017. PMID: 28887481 Free PMC article.
-
Reversal of fibrosis and portal hypertension by Empagliflozin treatment of CCl4-induced liver fibrosis: Emphasis on gal-1/NRP-1/TGF-β and gal-1/NRP-1/VEGFR2 pathways.Eur J Pharmacol. 2023 Nov 15;959:176066. doi: 10.1016/j.ejphar.2023.176066. Epub 2023 Sep 27. Eur J Pharmacol. 2023. PMID: 37769984
-
CD248/endosialin critically regulates hepatic stellate cell proliferation during chronic liver injury via a PDGF-regulated mechanism.Gut. 2016 Jul;65(7):1175-85. doi: 10.1136/gutjnl-2014-308325. Epub 2015 Jun 15. Gut. 2016. PMID: 26078290 Free PMC article.
-
Wnt signaling in liver fibrosis: progress, challenges and potential directions.Biochimie. 2013 Dec;95(12):2326-35. doi: 10.1016/j.biochi.2013.09.003. Epub 2013 Sep 13. Biochimie. 2013. PMID: 24036368 Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
XIAP Knockdown in Alcohol-Associated Liver Disease Models Exhibits Divergent in vitro and in vivo Phenotypes Owing to a Potential Zonal Inhibitory Role of SMAC.Front Physiol. 2021 May 7;12:664222. doi: 10.3389/fphys.2021.664222. eCollection 2021. Front Physiol. 2021. PMID: 34025452 Free PMC article.
-
Human airway smooth muscle cell proliferation from asthmatics is negatively regulated by semaphorin3A.Oncotarget. 2016 Dec 6;7(49):80238-80251. doi: 10.18632/oncotarget.12884. Oncotarget. 2016. PMID: 27791986 Free PMC article.
-
Neuropilin-1 aggravates liver cirrhosis by promoting angiogenesis via VEGFR2-dependent PI3K/Akt pathway in hepatic sinusoidal endothelial cells.EBioMedicine. 2019 May;43:525-536. doi: 10.1016/j.ebiom.2019.04.050. Epub 2019 May 3. EBioMedicine. 2019. PMID: 31060904 Free PMC article.
-
Neuropilin-1: A feasible link between liver pathologies and COVID-19.World J Gastroenterol. 2021 Jun 28;27(24):3516-3529. doi: 10.3748/wjg.v27.i24.3516. World J Gastroenterol. 2021. PMID: 34239266 Free PMC article. Review.
-
Exosome Adherence and Internalization by Hepatic Stellate Cells Triggers Sphingosine 1-Phosphate-dependent Migration.J Biol Chem. 2015 Dec 25;290(52):30684-96. doi: 10.1074/jbc.M115.671735. Epub 2015 Nov 3. J Biol Chem. 2015. PMID: 26534962 Free PMC article.
References
-
- Shah VH, Kamath PS. Portal hypertension and gastrointestinal bleeding. In Feldman M, Friedman LS, Brandt LJ, eds.Sleisenger and Fordtran’s Gastrointestinal and Liver Disease . 8th ed. Philadelphia, Pennsylvania, USA: Elsevier Health Sciences; 2006:1899–1934.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

