Umpolung reactivity in amide and peptide synthesis
- PMID: 20577205
- PMCID: PMC2945247
- DOI: 10.1038/nature09125
Umpolung reactivity in amide and peptide synthesis
Abstract
The amide bond is one of nature's most common functional and structural elements, as the backbones of all natural peptides and proteins are composed of amide bonds. Amides are also present in many therapeutic small molecules. The construction of amide bonds using available methods relies principally on dehydrative approaches, although oxidative and radical-based methods are representative alternatives. In nearly every example, carbon and nitrogen bear electrophilic and nucleophilic character, respectively, during the carbon-nitrogen bond-forming step. Here we show that activation of amines and nitroalkanes with an electrophilic iodine source can lead directly to amide products. Preliminary observations support a mechanism in which the polarities of the two reactants are reversed (German, umpolung) during carbon-nitrogen bond formation relative to traditional approaches. The use of nitroalkanes as acyl anion equivalents provides a conceptually innovative approach to amide and peptide synthesis, and one that might ultimately provide for efficient peptide synthesis that is fully reliant on enantioselective methods.
Figures

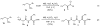
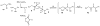
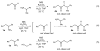

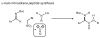
Comment in
-
Organic chemistry: Amide bonds made in reverse.Nature. 2010 Jun 24;465(7301):1020-2. doi: 10.1038/4651020a. Nature. 2010. PMID: 20577201 No abstract available.
References
-
- Valeur E, Bradley M. Amide bond formation: beyond the myth of coupling reagents. Chem Soc Rev. 2009;38:606–31. - PubMed
-
- Merrifield RB. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J Am Chem Soc. 1963;85:2149–54.
-
- Merrifield RB. Solid Phase Peptide Synthesis. II. The Synthesis of Bradykinin. J Am Chem Soc. 1964;86:304–5.
-
- Bode JW. Emerging methods in amide- and peptide-bond formation. Curr Opin Drug Disc Dev. 2006 Nov;9:765–75. - PubMed
-
- Saxon E, Armstrong JI, Bertozzi CR. A “traceless” Staudinger ligation for the chemoselective synthesis of amide bonds. Org Lett. 2000 Jul;2:2141–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

