Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design
- PMID: 20577269
- PMCID: PMC3167078
- DOI: 10.1038/nri2801
Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design
Abstract
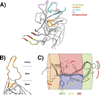
One of the main challenges of developing an HIV-1 vaccine lies in eliciting immune responses that can overcome the antigenic variability exhibited by HIV. Most HIV-1 vaccine development has focused on inducing immunity to conserved regions of the HIV-1 envelope. However, new studies of the sequence-variable regions of the HIV-1 gp120 envelope glycoprotein have shown that there are conserved immunological and structural features in these regions. Recombinant immunogens that include these features may provide the means to address the antigenic diversity of HIV-1 and induce protective antibodies that can prevent infection with HIV-1.
Figures


Similar articles
-
Rationally Designed Immunogens Targeting HIV-1 gp120 V1V2 Induce Distinct Conformation-Specific Antibody Responses in Rabbits.J Virol. 2016 Nov 28;90(24):11007-11019. doi: 10.1128/JVI.01409-16. Print 2016 Dec 15. J Virol. 2016. PMID: 27707920 Free PMC article.
-
Rationally Designed Vaccines Targeting the V2 Region of HIV-1 gp120 Induce a Focused, Cross-Clade-Reactive, Biologically Functional Antibody Response.J Virol. 2016 Nov 28;90(24):10993-11006. doi: 10.1128/JVI.01403-16. Print 2016 Dec 15. J Virol. 2016. PMID: 27630234 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
GP120: target for neutralizing HIV-1 antibodies.Annu Rev Immunol. 2006;24:739-69. doi: 10.1146/annurev.immunol.24.021605.090557. Annu Rev Immunol. 2006. PMID: 16551265 Review.
-
[VLP vaccines and effects of HIV-1 Env protein modifications on their antigenic properties].Mol Biol (Mosk). 2016 May-Jun;50(3):406-15. doi: 10.7868/S0026898416030113. Mol Biol (Mosk). 2016. PMID: 27414779 Review. Russian.
Cited by
-
Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial.PLoS One. 2013;8(1):e53629. doi: 10.1371/journal.pone.0053629. Epub 2013 Jan 17. PLoS One. 2013. PMID: 23349725 Free PMC article. Clinical Trial.
-
A DNA-based candidate HIV vaccine delivered via in vivo electroporation induces CD4 responses toward the α4β7-binding V2 loop of HIV gp120 in healthy volunteers.Clin Vaccine Immunol. 2012 Sep;19(9):1557-9. doi: 10.1128/CVI.00327-12. Epub 2012 Jul 25. Clin Vaccine Immunol. 2012. PMID: 22837097 Free PMC article. Clinical Trial.
-
An anti-HIV-1 V3 loop antibody fully protects cross-clade and elicits T-cell immunity in macaques mucosally challenged with an R5 clade C SHIV.PLoS One. 2011 Mar 31;6(3):e18207. doi: 10.1371/journal.pone.0018207. PLoS One. 2011. PMID: 21483815 Free PMC article.
-
Comprehensive Linear Epitope Prediction System for Host Specificity in Nodaviridae.Viruses. 2022 Jun 22;14(7):1357. doi: 10.3390/v14071357. Viruses. 2022. PMID: 35891339 Free PMC article.
-
Cross-clade HIV-1 neutralizing antibodies induced with V3-scaffold protein immunogens following priming with gp120 DNA.J Virol. 2011 Oct;85(19):9887-98. doi: 10.1128/JVI.05086-11. Epub 2011 Jul 27. J Virol. 2011. PMID: 21795338 Free PMC article.
References
-
- Gilbert PB, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–677. - PubMed
-
- Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
-
- Primer V, editor. IAVI Report. 2010. Understanding Antibody FUnctions: Beyond Neutralization.
-
- Starcich BR, et al. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986;45:637–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

