Palladium-Catalyzed Carboetherification and Carboamination Reactions of γ-Hydroxy- and γ-Aminoalkenes for the Synthesis of Tetrahydrofurans and Pyrrolidines
- PMID: 20577649
- PMCID: PMC2890284
Palladium-Catalyzed Carboetherification and Carboamination Reactions of γ-Hydroxy- and γ-Aminoalkenes for the Synthesis of Tetrahydrofurans and Pyrrolidines
Abstract
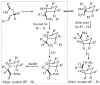
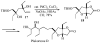
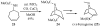
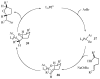
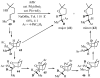
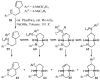
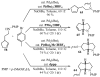
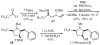
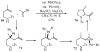
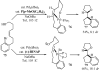
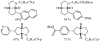
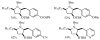
Substituted tetrahydrofuran and pyrrolidine moieties are displayed in a wide range of interesting biologically active molecules. The Pd-catalyzed carboetherification or carboamination of γ-hydroxy- and γ-aminoalkenes is a powerful tool for the construction of these heterocycles, as it is convergent and can allow access to a variety of analogs from a single γ-hydroxy- or γ-aminoalkene starting material. This microreview describes the current state of this field.
Figures
References
-
- Alali FQ, Liu X-X, McLaughlin JL. J. Nat. Prod. 1999;62:504–540. - PubMed
- Zafra-Polo MC, Figadere B, Gallardo T, Tormo JR, Cortes D. Phytochemistry. 1998;48:1087–1117.
-
- Faul MM, Huff BE. Chem. Rev. 2000;100:2407–2474. - PubMed
-
- Saleem M, Kim HJ, Ali MS, Lee YS. Nat. Prod. Rep. 2005:696–716. - PubMed
-
- Kang EJ, Lee E. Chem. Rev. 2005;105:4348–4378. - PubMed
-
- O’Hagen D. Nat. Prod. Rep. 2000;17:435–446. - PubMed
- Mitchinson A, Nadin A. J. Chem. Soc., Perkin Trans. 1. 2000:2862–2892.
- Massiot G, Delaude C. Alkaloids. 1986;27:269–322.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources