Methods of assessing vagus nerve activity and reflexes
- PMID: 20577901
- PMCID: PMC4322860
- DOI: 10.1007/s10741-010-9174-6
Methods of assessing vagus nerve activity and reflexes
Abstract
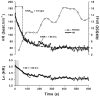
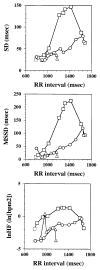
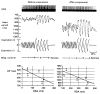
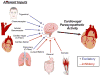
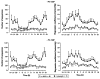
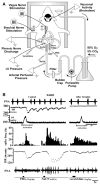
The methods used to assess cardiac parasympathetic (cardiovagal) activity and its effects on the heart in both humans and animal models are reviewed. Heart rate (HR)-based methods include measurements of the HR response to blockade of muscarinic cholinergic receptors (parasympathetic tone), beat-to-beat HR variability (HRV) (parasympathetic modulation), rate of post-exercise HR recovery (parasympathetic reactivation), and reflex-mediated changes in HR evoked by activation or inhibition of sensory (afferent) nerves. Sources of excitatory afferent input that increase cardiovagal activity and decrease HR include baroreceptors, chemoreceptors, trigeminal receptors, and subsets of cardiopulmonary receptors with vagal afferents. Sources of inhibitory afferent input include pulmonary stretch receptors with vagal afferents and subsets of visceral and somatic receptors with spinal afferents. The different methods used to assess cardiovagal control of the heart engage different mechanisms, and therefore provide unique and complementary insights into underlying physiology and pathophysiology. In addition, techniques for direct recording of cardiovagal nerve activity in animals; the use of decerebrate and in vitro preparations that avoid confounding effects of anesthesia; cardiovagal control of cardiac conduction, contractility, and refractoriness; and noncholinergic mechanisms are described. Advantages and limitations of the various methods are addressed, and future directions are proposed.
Figures


References
-
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ for the ATRAMI investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. - PubMed
-
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. - PubMed
-
- Katona PG, Lipson D, Dauchot PJ. Opposing central and peripheral effects of atropine on parasympathetic cardiac control. Am J Physiol Heart. 1977;232:H146–H151. - PubMed
-
- Brodde OE, Bruck H, Leineweber K, Seyfarth T. Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart. Basic Res Cardiol. 2001;96:528–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

