Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins
- PMID: 20578001
- PMCID: PMC2894268
- DOI: 10.1002/bip.21483
Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins
Abstract
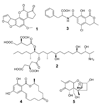
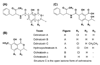
Polyketides (PKs) are a large group of natural products produced by microorganisms and plants. They are biopolymers of acetate and other short carboxylates and are biosynthesized by multifunctional enzymes called polyketide synthases (PKSs). This review discusses the biosynthesis of four toxic PK, aflatoxins, fumonisins, ochratoxins (OTs), and zearalenone. These metabolites are structurally diverse and differ in their mechanisms of toxicity. However, they are all of concern in food safety and agriculture because of their toxic properties and their frequent accumulation in crops used for food and feed. The focus is on the recent advancements in the understanding of the molecular mechanisms for the biosynthesis of these mycotoxins. Several of the mycotoxin PKSs have been genetically and biochemically studied while other PKSs remain to be investigated. Multiple post-PKS modifications are often required for the maturation of the mycotoxins. Many of these modification steps for aflatoxins and fumonisins are well established while the post-PKS modifications for zearalenone and OTs remain to be biochemically characterized. More efforts are needed to completely illustrate the biosynthetic mechanisms for this important group of PKs.
Copyright 2010 Wiley Periodicals, Inc.
Figures




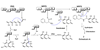

Similar articles
-
Molecular biology of mycotoxin biosynthesis.FEMS Microbiol Lett. 1999 Jun 15;175(2):149-63. doi: 10.1111/j.1574-6968.1999.tb13614.x. FEMS Microbiol Lett. 1999. PMID: 10386364 Review.
-
Phylogenetic study of polyketide synthases and nonribosomal peptide synthetases involved in the biosynthesis of mycotoxins.Toxins (Basel). 2013 Apr 19;5(4):717-42. doi: 10.3390/toxins5040717. Toxins (Basel). 2013. PMID: 23604065 Free PMC article.
-
Engineered biosynthesis of regioselectively modified aromatic polyketides using bimodular polyketide synthases.PLoS Biol. 2004 Feb;2(2):E31. doi: 10.1371/journal.pbio.0020031. Epub 2004 Feb 17. PLoS Biol. 2004. PMID: 14966533 Free PMC article.
-
Chemical and biological approaches for mycotoxin control: a review.Recent Pat Food Nutr Agric. 2009 Jun;1(2):155-61. doi: 10.2174/2212798410901020155. Recent Pat Food Nutr Agric. 2009. PMID: 20653536 Review.
-
Insights into natural products biosynthesis from analysis of 490 polyketide synthases from Fusarium.Fungal Genet Biol. 2016 Apr;89:37-51. doi: 10.1016/j.fgb.2016.01.008. Epub 2016 Jan 27. Fungal Genet Biol. 2016. PMID: 26826610
Cited by
-
Exposure Assessment of Aflatoxin B1 through Consumption of Rice in the United Arab Emirates.Int J Environ Res Public Health. 2022 Nov 15;19(22):15000. doi: 10.3390/ijerph192215000. Int J Environ Res Public Health. 2022. PMID: 36429720 Free PMC article.
-
Unveiling toxigenic Fusarium species causing maize ear rot: insights into fumonisin production potential.Front Plant Sci. 2025 Mar 21;16:1516644. doi: 10.3389/fpls.2025.1516644. eCollection 2025. Front Plant Sci. 2025. PMID: 40190660 Free PMC article.
-
Characterization of tea (Camellia sinensis L.) flower extract and insights into its antifungal susceptibilities of Aspergillus flavus.BMC Complement Med Ther. 2023 Aug 14;23(1):286. doi: 10.1186/s12906-023-04122-5. BMC Complement Med Ther. 2023. PMID: 37580785 Free PMC article.
-
Sequencing and functional annotation of the whole genome of the filamentous fungus Aspergillus westerdijkiae.BMC Genomics. 2016 Aug 15;17(1):633. doi: 10.1186/s12864-016-2974-x. BMC Genomics. 2016. PMID: 27527502 Free PMC article.
-
Research in bioanalysis and separations at the University of Nebraska - Lincoln.Bioanalysis. 2011 May;3(10):1065-76. doi: 10.4155/bio.11.64. Bioanalysis. 2011. PMID: 21585300 Free PMC article.
References
-
- Brase S, Encinas A, Keck J, Nising CF. Chem Rev. 2009;109:3903–3990. - PubMed
-
- Richard JL. Int J Food Microbiol. 2007;119:3–10. - PubMed
-
- Richard JL. International Journal of Food Microbiology. 2007;119:3–10. - PubMed
-
- Binder EM, Tan LM, Chin LJ, Handl J, Richard J. Animal Feed Science and Technology. 2007;137:265–282.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

