Aquaporin-1 facilitates angiogenic invasion in the pathological neovasculature that accompanies cirrhosis
- PMID: 20578142
- PMCID: PMC2928054
- DOI: 10.1002/hep.23628
Aquaporin-1 facilitates angiogenic invasion in the pathological neovasculature that accompanies cirrhosis
Abstract
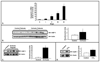
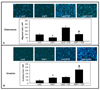
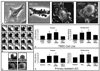
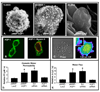
Increasing evidence suggests that hepatic fibrosis and pathological angiogenesis are interdependent processes that occur in parallel. Endothelial cell invasion is requisite for angiogenesis, and thus studies of the mechanisms governing liver endothelial cell (LEC) invasion during cirrhosis are of great importance. Emerging research implicates amoeboid-type motility and membrane blebbing as features that may facilitate invasion through matrix-rich microenvironments. Aquaporins (AQPs) are integral membrane water channels, recognized for their importance in epithelial secretion and absorption. However, recent studies also suggest links between water transport and cell motility or invasion. Therefore, the purpose of this study was to test the hypothesis that AQP-1 is involved in amoeboid motility and angiogenic invasion during cirrhosis. AQP-1 expression and localization was examined in normal and cirrhotic liver tissues derived from human and mouse. AQP-1 levels were modulated in LEC using retroviral overexpression or small interfering RNA (siRNA) knockdown and functional effects on invasion, membrane blebbing dynamics, and osmotic water permeability were assayed. Results demonstrate that AQP-1 is up-regulated in the small, angiogenic, neovasculature within the fibrotic septa of cirrhotic liver. AQP-1 overexpression promotes fibroblast growth factor (FGF)-induced dynamic membrane blebbing in LEC, which is sufficient to augment invasion through extracellular matrix. Additionally, AQP-1 localizes to plasma membrane blebs, where it increases osmotic water permeability and locally facilitates the rapid, trans-membrane flux of water.
Conclusion: AQP-1 enhances osmotic water permeability and FGF-induced dynamic membrane blebbing in LEC and thereby drives invasion and pathological angiogenesis during cirrhosis.
Figures




References
-
- Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733–746. vii. - PubMed
-
- Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620. - PubMed
-
- Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, Friedman SL, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–1926. - PubMed
-
- Morales-Ruiz M, Jimenez W. Neovascularization, angiogenesis and vascular remodeling in portal hypertension. In: Sanyal A, Shah V, editors. Portal Hypertension: Pathobiology, Evaluation, and Treatment. Totowa, NJ: Humana Press; 2005. pp. 99–112.
-
- Fabris L, Cadamuro M, Libbrecht L, Raynaud P, Spirli C, Fiorotto R, Okolicsanyi L, et al. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology. 2008;47:719–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
