Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer
- PMID: 20578239
- PMCID: PMC2932466
- DOI: 10.1002/jcp.22196
Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer
Abstract
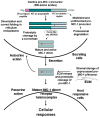
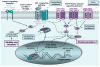
Multifunctional macrophage inhibitory cytokine-1, MIC-1, is a member of the transforming growth factor-beta (TGF-beta) superfamily that plays key roles in the prenatal development and regulation of the cellular responses to stress signals and inflammation and tissue repair after acute injuries in adult life. The stringent control of the MIC-1 expression, secretion, and functions involves complex regulatory mechanisms and the interplay of other growth factor signaling networks that control the cell behavior. The deregulation of MIC-1 expression and signaling pathways has been associated with diverse human diseases and cancer progression. The MIC-1 expression levels substantially increase in cancer cells, serum, and/or cerebrospinal fluid during the progression of diverse human aggressive cancers, such as intracranial brain tumors, melanoma, and lung, gastrointestinal, pancreatic, colorectal, prostate, and breast epithelial cancers. Of clinical interest, an enhanced MIC-1 expression has been positively correlated with poor prognosis and patient survival. Secreted MIC-1 cytokine, like the TGF-beta prototypic member of the superfamily, may provide pleiotropic roles in the early and late stages of carcinogenesis. In particular, MIC-1 may contribute to the proliferation, migration, invasion, metastases, and treatment resistance of cancer cells as well as tumor-induced anorexia and weight loss in the late stages of cancer. Thus, secreted MIC-1 cytokine constitutes a new potential biomarker and therapeutic target of great clinical interest for the development of novel diagnostic and prognostic methods and/or cancer treatment against numerous metastatic, recurrent, and lethal cancers.
(c) 2010 Wiley-Liss, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Akiyama M, Okano K, Fukada Y, Okano T. Macrophage inhibitory cytokine MIC-1 is upregulated by short-wavelength light in cultured normal human dermal fibroblasts. FEBS Lett. 2009;583:933–937. - PubMed
-
- Albertoni M, Shaw PH, Nozaki M, Godard S, Tenan M, Hamou MF, Fairlie DW, Breit SN, Paralkar VM, de Tribolet N, Van Meir EG, Hegi ME. Anoxia induces macrophage inhibitory cytokine-1 (MIC-1) in glioblastoma cells independently of p53 and HIF-1. Oncogene. 2002;21:4212–4219. - PubMed
-
- Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59:901–908. - PubMed
-
- Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol. 2005;67:356–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

