Investigating gradients of gene expression involved in early human cortical development
- PMID: 20579172
- PMCID: PMC2992409
- DOI: 10.1111/j.1469-7580.2010.01259.x
Investigating gradients of gene expression involved in early human cortical development
Abstract
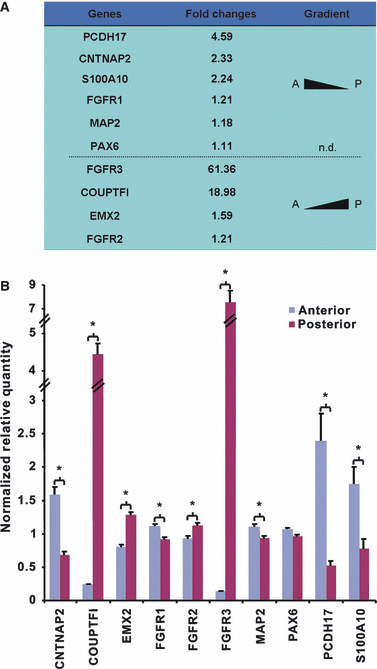
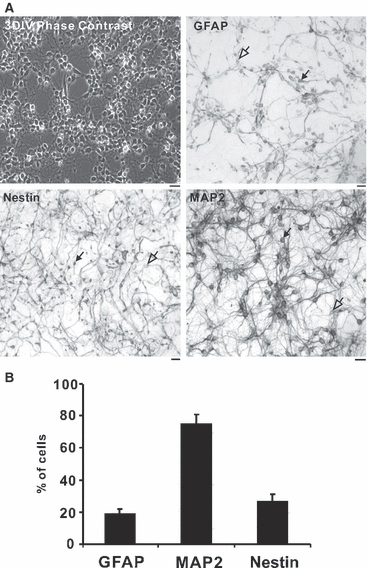
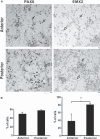
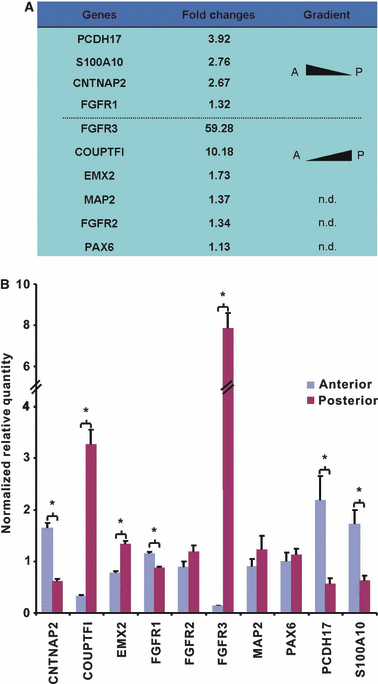
The division of the neocortex into functional areas (the cortical map) differs little between individuals, although brain lesions in development can lead to substantial re-organization of regional identity. We are studying how the cortical map is established in the human brain as a first step towards understanding this plasticity. Previous work on rodent development has identified certain transcription factors (e.g. Pax6, Emx2) expressed in gradients across the neocortex that appear to control regional expression of cell adhesion molecules and organization of area-specific thalamocortical afferent projections. Although mechanisms may be shared, the human neocortex is composed of different and more complex local area identities. Using Affymetrix gene chips of human foetal brain tissue from 8 to 12.5 post-conceptional weeks [PCW, equivalent to Carnegie stage (CS) 23, to Foetal stage (F) 4], human material obtained from the MRC-Wellcome Trust Human Developmental Biology Resource (http://www.hdbr.org), we have identified a number of genes that exhibit gradients along the anterior-posterior axis of the neocortex. Gene probe sets that were found to be upregulated posteriorally compared to anteriorally, included EMX2, COUPTFI and FGF receptor 3, and those upregulated anteriorally included cell adhesion molecules such as cadherins and protocadherins, as well as potential motor cortex markers and frontal markers (e.g. CNTNAP2, PCDH17, ROBO1, and CTIP2). Confirmation of graded expression for a subset of these genes was carried out using real-time PCR. Furthermore, we have established a dissociation cell culture model utilizing tissue dissected from anteriorally or posteriorally derived developing human neocortex that exhibits similar gradients of expression of these genes for at least 72 h in culture.
© 2010 The Authors. Journal of Anatomy © 2010 Anatomical Society of Great Britain and Ireland.
Figures

References
-
- Arlotta P, Molyneaux BJ, Chen J, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Basu A, Graziadio S, Smith M, et al. Developmental plasticity connects visual cortex to motoneurons after stroke. Ann Neurol. 2010;67:132–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

