EBM, HTA, and CER: clearing the confusion
- PMID: 20579285
- PMCID: PMC2980346
- DOI: 10.1111/j.1468-0009.2010.00598.x
EBM, HTA, and CER: clearing the confusion
Abstract
Context: The terms evidence-based medicine (EBM), health technology assessment (HTA), comparative effectiveness research (CER), and other related terms lack clarity and so could lead to miscommunication, confusion, and poor decision making. The objective of this article is to clarify their definitions and the relationships among key terms and concepts.
Methods: This article used the relevant methods and policy literature as well as the websites of organizations engaged in evidence-based activities to develop a framework to explain the relationships among the terms EBM, HTA, and CER.
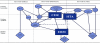
Findings: This article proposes an organizing framework and presents a graphic demonstrating the differences and relationships among these terms and concepts.
Conclusions: More specific terminology and concepts are necessary for an informed and clear public policy debate. They are even more important to inform decision making at all levels and to engender more accountability by the organizations and individuals responsible for these decisions.
Figures

References
-
- AMCP (Academy of Managed Care Pharmacy) The AMCP Format for Formulary Submissions. 2009. Version 3.0. Available at http://www.fmcpnet.org/index.cfm?c=news.details&a=wn&id=C604C25D_2_1~Fin... (accessed November 25, 2009).
-
- American College of Physicians. Information on Cost-Effectiveness: An Essential Product of a National Comparative Effectiveness Program. Annals of Internal Medicine. 2008;148:956–61. - PubMed
-
- Blue Cross Blue Shield. Technology Evaluation Center. 2009. Available at http://www.bcbs.com/blueresources/tec (accessed November 25, 2009).
-
- CADTH (Canadian Agency for Drugs and Technologies in Health) Common Drug Review. 2009. Available at http://www.cadth.ca/index.php/en/cdr (accessed November 25, 2009).
-
- Center for Evidence-Based Policy. The Drug Effectiveness Review Project (DERP) 2010. Available at http://www.ohsu.edu/ohsuedu/research/policycenter/ (accessed February 24, 2010).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

