Delta opioid receptor on equine sperm cells: subcellular localization and involvement in sperm motility analyzed by computer assisted sperm analyzer (CASA)
- PMID: 20579355
- PMCID: PMC2901311
- DOI: 10.1186/1477-7827-8-78
Delta opioid receptor on equine sperm cells: subcellular localization and involvement in sperm motility analyzed by computer assisted sperm analyzer (CASA)
Abstract
Background: Opioid receptors and endogenous opioid peptides act not only in the control of nociceptive pathways, indeed several reports demonstrate the effects of opiates on sperm cell motility and morphology suggesting the importance of these receptors in the modulation of reproduction in mammals. In this study we investigated the expression of delta opioid receptors on equine spermatozoa by western blot/indirect immunofluorescence and its relationship with sperm cell physiology.
Methods: We analyzed viability, motility, capacitation, acrosome reaction and mitochondrial activity in the presence of naltrindole and DPDPE by means of a computer assisted sperm analyzer and a fluorescent confocal microscope. The evaluation of viability, capacitation and acrosome reaction was carried out by the double CTC/Hoechst staining, whereas mitochondrial activity was assessed by means of MitoTracker Orange dye.
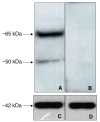
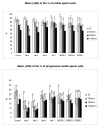
Results: We showed that in equine sperm cells, delta opioid receptor is expressed as a doublet of 65 and 50 kDa molecular mass and is localized in the mid piece of tail; we also demonstrated that naltrindole, a delta opioid receptor antagonist, could be utilized in modulating several physiological parameters of the equine spermatozoon in a dose-dependent way. We also found that low concentrations of the antagonist increase sperm motility whereas high concentrations show the opposite effect. Moreover low concentrations hamper capacitation, acrosome reaction and viability even if the percentage of cells with active mitochondria seems to be increased; the opposite effect is exerted at high concentrations. We have also observed that the delta opioid receptor agonist DPDPE is scarcely involved in affecting the same parameters at the employed concentrations.
Conclusions: The results described in this paper add new important details in the comprehension of the mammalian sperm physiology and suggest new insights for improving reproduction and for optimizing equine breeding.
Figures


Similar articles
-
Expression and localization of delta-, kappa-, and mu-opioid receptors in human spermatozoa and implications for sperm motility.J Clin Endocrinol Metab. 2006 Dec;91(12):4969-75. doi: 10.1210/jc.2006-0599. Epub 2006 Sep 19. J Clin Endocrinol Metab. 2006. PMID: 16984994
-
Expression and subcellular localization of the mu-opioid receptor in equine spermatozoa: evidence for its functional role.Reproduction. 2005 Jan;129(1):39-49. doi: 10.1530/rep.1.00284. Reproduction. 2005. PMID: 15615897
-
Delta and kappa opioid receptors on mouse sperm cells: Expression, localization and involvement on in vitro fertilization.Reprod Toxicol. 2020 Apr;93:211-218. doi: 10.1016/j.reprotox.2020.02.013. Epub 2020 Mar 4. Reprod Toxicol. 2020. PMID: 32145291
-
The mu (μ) and delta (δ) opioid receptors modulate boar sperm motility.Mol Reprod Dev. 2016 Aug;83(8):724-34. doi: 10.1002/mrd.22675. Mol Reprod Dev. 2016. PMID: 27391529
-
Effect of leptin on motility, capacitation and acrosome reaction of human spermatozoa.Int J Androl. 2009 Dec;32(6):687-94. doi: 10.1111/j.1365-2605.2008.00931.x. Epub 2008 Dec 2. Int J Androl. 2009. PMID: 19076257
Cited by
-
Regulation of male fertility by the opioid system.Mol Med. 2011;17(7-8):846-53. doi: 10.2119/molmed.2010.00268. Epub 2011 Mar 16. Mol Med. 2011. PMID: 21431247 Free PMC article. Review.
-
Presence and Location of CatSper 1-4, Opioid (μ, δ and κ) and CD44 Receptors in SPERMATOZOA from AOUDAD, IBERIAN IBEX and Mouflon.Vet Med Sci. 2025 Jul;11(4):e70459. doi: 10.1002/vms3.70459. Vet Med Sci. 2025. PMID: 40679138 Free PMC article.
-
The effects of morphine abuse on sperm parameters, chromatin integrity and apoptosis in men.JBRA Assist Reprod. 2022 Aug 4;26(3):444-449. doi: 10.5935/1518-0557.20210110. JBRA Assist Reprod. 2022. PMID: 34995046 Free PMC article.
-
Mu opioid receptor in spermatozoa, eggs and larvae of gilthead sea bream (Sparus Aurata) and its involvement in stress related to aquaculture.Fish Physiol Biochem. 2014 Aug;40(4):997-1009. doi: 10.1007/s10695-013-9900-9. Epub 2013 Dec 15. Fish Physiol Biochem. 2014. PMID: 24338156
-
Consequences of Parental Opioid Exposure on Neurophysiology, Behavior, and Health in the Next Generations.Cold Spring Harb Perspect Med. 2021 Oct 1;11(10):a040436. doi: 10.1101/cshperspect.a040436. Cold Spring Harb Perspect Med. 2021. PMID: 32601130 Free PMC article. Review.
References
-
- Elde R, Hökfelt T. In: Handbook of experimental pharmacology, Opioids. Herz A, editor. Vol. 1. Berlin: Springer; 1993. Coexistence of opioid peptides with other neurotransmitters; pp. 585–624.
-
- Bostwick DG, Null WE, Holmes D, Weber E, Barchas JD, Bensch KG. Expression of opioid peptides in tumors. New Engl J Med. 1987;317:1439–1443. - PubMed
-
- Fabbri A, Jannini EA, Gnessi L, Ulisse S, Moretti C, Isidori A. Neuroendocrine control of male reproductive function. The opioid system as a model of control at multiple sites. J Steroid Biochem. 1986;32(Suppl 1B):145–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

