gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression
- PMID: 20579975
- PMCID: PMC2930197
- DOI: 10.1016/j.biopsych.2010.04.024
gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression
Erratum in
- Biol Psychiatry. 2012 Dec 1;72(11):978. Dosage error in article text
Abstract
Background: The gamma-aminobutyric acid (GABA) Type A receptor deficits that are induced by global or forebrain-specific heterozygous inactivation of the gamma2 subunit gene in mouse embryos result in behavior indicative of trait anxiety and depressive states. By contrast, a comparable deficit that is delayed to adolescence is without these behavioral consequences. Here we characterized gamma2-deficient mice with respect to hypothalamic-pituitary-adrenal (HPA) axis abnormalities and antidepressant drug responses.
Methods: We analyzed the behavioral responses of gamma2(+/-) mice to desipramine and fluoxetine in novelty suppressed feeding, forced swim, tail suspension, and sucrose consumption tests as well as GABA(A) receptor deficit- and antidepressant drug treatment-induced alterations in serum corticosterone.
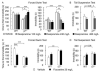
Results: Baseline corticosterone concentrations in adult gamma2-deficient mice were elevated independent of whether the genetic lesion was induced during embryogenesis or delayed to adolescence. However, the manifestation of anxious-depressive behavior in different gamma2-deficient mouse lines was correlated with early onset HPA axis hyperactivity during postnatal development. Chronic but not subchronic treatment of gamma2(+/-) mice with fluoxetine or desipramine normalized anxiety-like behavior in the novelty suppressed feeding test. Moreover, desipramine had antidepressant-like effects in that it normalized HPA axis function and depression-related behavior of gamma2(+/-) mice in the forced swim, tail suspension, and sucrose consumption tests. By contrast, fluoxetine was ineffective as an antidepressant and failed to normalize HPA axis function.
Conclusions: Developmental deficits in GABAergic inhibition in the forebrain cause behavioral and endocrine abnormalities and selective antidepressant drug responsiveness indicative of anxious-depressive disorders such as melancholic depression, which are frequently characterized by HPA axis hyperactivity and greater efficacy of desipramine versus fluoxetine.
2010 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
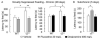
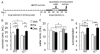

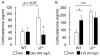
Comment in
-
Studies in genetically modified mice suggest novel mechanisms of mood regulation.Biol Psychiatry. 2010 Sep 15;68(6):500-2. doi: 10.1016/j.biopsych.2010.07.020. Biol Psychiatry. 2010. PMID: 20797461 Free PMC article. No abstract available.
References
-
- Kendler KS. Major depression and generalised anxiety disorder. Same genes, (partly)different environments--revisited. Br J Psychiatry Suppl. 1996:68–75. - PubMed
-
- Eley TC, Bolton D, O'Connor TG, Perrin S, Smith P, Plomin R. A twin study of anxiety-related behaviours in pre-school children. J Child Psychol Psychiatry. 2003;44:945–960. - PubMed
-
- Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):69–76. - PubMed
-
- Murphy JM, Horton NJ, Laird NM, Monson RR, Sobol AM, Leighton AH. Anxiety and depression: a 40-year perspective on relationships regarding prevalence, distribution, and comorbidity. Acta Psychiatr Scand. 2004;109:355–375. - PubMed
-
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165:342–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

