Age-related neuronal loss in the cochlea is not delayed by synaptic modulation
- PMID: 20580130
- PMCID: PMC2947614
- DOI: 10.1016/j.neurobiolaging.2010.05.011
Age-related neuronal loss in the cochlea is not delayed by synaptic modulation
Abstract
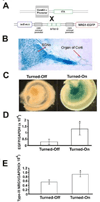
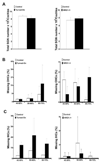
Age-related synaptic change is associated with the functional decline of the nervous system. It is unknown whether this synaptic change is the cause or the consequence of neuronal cell loss. We have addressed this question by examining mice genetically engineered to over- or underexpress neuregulin-1 (NRG1), a direct modulator of synaptic transmission. Transgenic mice overexpressing NRG1 in spiral ganglion neurons (SGNs) showed improvements in hearing thresholds, whereas NRG1 -/+ mice show a complementary worsening of thresholds. However, no significant change in age-related loss of SGNs in either NRG1 -/+ mice or mice overexpressing NRG1 was observed, while a negative association between NRG1 expression level and survival of inner hair cells during aging was observed. Subsequent studies provided evidence that modulating NRG1 levels changes synaptic transmission between SGNs and hair cells. One of the most dramatic examples of this was the reversal of lower hearing thresholds by "turning-off" NRG1 overexpression. These data demonstrate for the first time that synaptic modulation is unable to prevent age-related neuronal loss in the cochlea.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures






References
-
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. - PubMed
-
- Bao J. Signal transduction by the cytoplasmic domain of Neuregulin-1 and its roles in neuronal aging. Curr. Signal. Trans. Therapy. 2007;2:240–245.
-
- Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat. Neurosci. 2004;7:1250–1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

