Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium
- PMID: 20580721
- PMCID: PMC2930126
- DOI: 10.1053/j.gastro.2010.05.038
Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium
Abstract
Background & aims: Factors that regulate enterocyte apoptosis in necrotizing enterocolitis (NEC) remain incompletely understood, although Toll-like receptor-4 (TLR4) signaling in enterocytes plays a major role. Nucleotide-binding oligomerization domain-2 (NOD2) is an immune receptor that regulates other branches of the immune system, although its effects on TLR4 in enterocytes and its role in NEC remain unknown. We now hypothesize that activation of NOD2 in the newborn intestine inhibits TLR4, and that failure of NOD2 signaling leads to NEC through increased TLR4-mediated enterocyte apoptosis.
Methods: The effects of NOD2 on enterocyte TLR4 signaling and intestinal injury and repair were assessed in enterocytes lacking TLR4 or NOD2, in mice with intestinal-specific wild-type or dominant-negative TLR4 or NOD2, and in mice with NEC. A protein array was performed on NOD2-activated enterocytes to identify novel effector molecules involved.
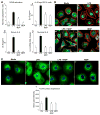
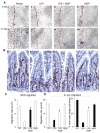
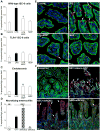
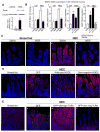
Results: TLR4 activation caused apoptosis in newborn but not adult small intestine or colon, and its intestinal expression was influenced by NOD2. NOD2 activation inhibited TLR4 in enterocytes, but not macrophages, and reversed the effects of TLR4 on intestinal mucosal injury and repair. Protection from TLR4-induced enterocyte apoptosis by NOD2 required a novel pathway linking NOD2 with the apoptosis mediator second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low PI (SMAC-DIABLO), both in vitro and in vivo. Strikingly, activation of NOD2 reduced SMAC-DIABLO expression, attenuated the extent of enterocyte apoptosis, and reduced the severity of NEC.
Conclusions: These findings reveal a novel inhibitory interaction between TLR4 and NOD2 signaling in enterocytes leading to the regulation of enterocyte apoptosis and suggest a therapeutic role for NOD2 in the protection of intestinal diseases such as NEC.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis.Gastroenterology. 2010 Jan;138(1):185-96. doi: 10.1053/j.gastro.2009.09.045. Epub 2009 Sep 26. Gastroenterology. 2010. PMID: 19786028 Free PMC article.
-
The human milk oligosaccharides 2'-fucosyllactose and 6'-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling.Pediatr Res. 2021 Jan;89(1):91-101. doi: 10.1038/s41390-020-0852-3. Epub 2020 Mar 27. Pediatr Res. 2021. PMID: 32221473 Free PMC article.
-
A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair.J Immunol. 2007 Oct 1;179(7):4808-20. doi: 10.4049/jimmunol.179.7.4808. J Immunol. 2007. PMID: 17878380
-
Toll-Like Receptor-Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis.Cell Mol Gastroenterol Hepatol. 2018 Apr 6;6(2):229-238.e1. doi: 10.1016/j.jcmgh.2018.04.001. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30105286 Free PMC article. Review.
-
Innate immune signaling in the pathogenesis of necrotizing enterocolitis.Clin Dev Immunol. 2013;2013:475415. doi: 10.1155/2013/475415. Epub 2013 May 23. Clin Dev Immunol. 2013. PMID: 23762089 Free PMC article. Review.
Cited by
-
Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch.Semin Pediatr Surg. 2013 May;22(2):76-82. doi: 10.1053/j.sempedsurg.2013.01.003. Semin Pediatr Surg. 2013. PMID: 23611610 Free PMC article. Review.
-
The immunological landscape in necrotising enterocolitis.Expert Rev Mol Med. 2016 Jun 24;18:e12. doi: 10.1017/erm.2016.13. Expert Rev Mol Med. 2016. PMID: 27341512 Free PMC article. Review.
-
Bifidobacterium: Host-Microbiome Interaction and Mechanism of Action in Preventing Common Gut-Microbiota-Associated Complications in Preterm Infants: A Narrative Review.Nutrients. 2023 Jan 30;15(3):709. doi: 10.3390/nu15030709. Nutrients. 2023. PMID: 36771414 Free PMC article. Review.
-
Toll-like receptor-4 in human and mouse colonic epithelium is developmentally regulated: a possible role in necrotizing enterocolitis.Pediatr Res. 2015 Mar;77(3):416-24. doi: 10.1038/pr.2014.207. Epub 2014 Dec 18. Pediatr Res. 2015. PMID: 25521917 Free PMC article.
-
NOD2 deficiency increases retrograde transport of secretory IgA complexes in Crohn's disease.Nat Commun. 2021 Jan 11;12(1):261. doi: 10.1038/s41467-020-20348-0. Nat Commun. 2021. PMID: 33431850 Free PMC article.
References
-
- Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49–57. - PubMed
-
- Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622–9. - PubMed
-
- Leaphart CL, Cavallo JC, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–4820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

