Engraftment of cells from porcine islets of Langerhans and normalization of glucose tolerance following transplantation of pig pancreatic primordia in nonimmune-suppressed diabetic rats
- PMID: 20581052
- PMCID: PMC2913376
- DOI: 10.2353/ajpath.2010.091193
Engraftment of cells from porcine islets of Langerhans and normalization of glucose tolerance following transplantation of pig pancreatic primordia in nonimmune-suppressed diabetic rats
Abstract
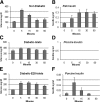
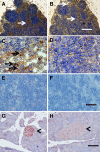
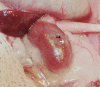
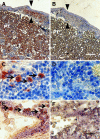
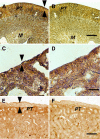
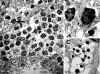
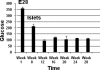
Transplantation therapy for human diabetes is limited by the toxicity of immunosuppressive drugs. However, even if toxicity can be minimalized, there will still be a shortage of human donor organs. Xenotransplantation of porcine islets may be a strategy to overcome these supply problems. Xenotransplantation in mesentery of pig pancreatic primordia obtained very early during organogenesis [embryonic day 28 (E28)] can obviate the need for immune suppression in rats or rhesus macaques. Here, in rats transplanted previously with E28 pig pancreatic primordia in the mesentery, we show normalization of glucose tolerance in nonimmune-suppressed streptozotocin-diabetic LEW rats and insulin and porcine proinsulin mRNA-expressing cell engraftment in the kidney following implantation of porcine islets beneath the renal capsule. Donor cell engraftment was confirmed using fluorescent in situ hybridization for the porcine X chromosome and electron microscopy. In contrast, cells from islets did not engraft in the kidney without prior transplantation of E28 pig pancreatic primordia in the mesentery. This is the first report of prolonged engraftment and sustained normalization of glucose tolerance following transplantation of porcine islets in nonimmune-suppressed, immune-competent rodents. The data are consistent with tolerance induction to a cell component of porcine islets induced by previous transplantation of E28 pig pancreatic primordia.
Figures



Similar articles
-
Development of a novel xenotransplantation strategy for treatment of diabetes mellitus in rat hosts and translation to non-human primates.Organogenesis. 2012 Apr-Jun;8(2):41-8. doi: 10.4161/org.20930. Epub 2012 Apr 1. Organogenesis. 2012. PMID: 22699748 Free PMC article. Review.
-
Engraftment of cells from porcine islets of Langerhans following transplantation of pig pancreatic primordia in non-immunosuppressed diabetic rhesus macaques.Organogenesis. 2011 Jul-Sep;7(3):154-62. doi: 10.4161/org.7.3.16522. Epub 2011 Jul 1. Organogenesis. 2011. PMID: 21654197 Free PMC article.
-
Long-term engraftment following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic rhesus macaques.Xenotransplantation. 2007 Nov;14(6):591-602. doi: 10.1111/j.1399-3089.2007.00429.x. Xenotransplantation. 2007. PMID: 17991147
-
Glucose tolerance normalization following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic ZDF rats.Transpl Immunol. 2006 Nov;16(3-4):176-84. doi: 10.1016/j.trim.2006.08.007. Epub 2006 Sep 7. Transpl Immunol. 2006. PMID: 17138051
-
Organogenetic tolerance.Organogenesis. 2010 Oct-Dec;6(4):270-5. doi: 10.4161/org.6.4.13283. Organogenesis. 2010. PMID: 21220963 Free PMC article. Review.
Cited by
-
Development of a novel xenotransplantation strategy for treatment of diabetes mellitus in rat hosts and translation to non-human primates.Organogenesis. 2012 Apr-Jun;8(2):41-8. doi: 10.4161/org.20930. Epub 2012 Apr 1. Organogenesis. 2012. PMID: 22699748 Free PMC article. Review.
-
Xenotransplantation of embryonic pig pancreas for treatment of diabetes mellitus in non-human primates.J Biomed Sci Eng. 2013 May 1;6(5A):10.4236/jbise.2013.65A002. doi: 10.4236/jbise.2013.65A002. J Biomed Sci Eng. 2013. PMID: 24312695 Free PMC article.
-
Classic and current opinion in embryonic organ transplantation.Curr Opin Organ Transplant. 2014 Apr;19(2):133-9. doi: 10.1097/MOT.0000000000000054. Curr Opin Organ Transplant. 2014. PMID: 24535425 Free PMC article. Review.
-
Xenotransplantation of embryonic pig kidney or pancreas to replace the function of mature organs.J Transplant. 2011;2011:501749. doi: 10.1155/2011/501749. Epub 2010 Dec 28. J Transplant. 2011. PMID: 21234246 Free PMC article.
-
Engraftment of cells from porcine islets of Langerhans following transplantation of pig pancreatic primordia in non-immunosuppressed diabetic rhesus macaques.Organogenesis. 2011 Jul-Sep;7(3):154-62. doi: 10.4161/org.7.3.16522. Epub 2011 Jul 1. Organogenesis. 2011. PMID: 21654197 Free PMC article.
References
-
- Groth CG, Korsgren O, Tibell A, Tollemar J, Moller E, Bolinder J, Ostman J, Reinholt FR, Hellerstrom C, Andersson A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–1404. - PubMed
-
- Hering B, Wijkstrom M, Graham M, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, Christians U, Finnegan C, Mills CD, Sutherland DE, Bansal-Pakala P, Murtaugh MP, Kirchhof N, Schuurman HJ. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. - PubMed
-
- Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Lahorn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, Pearson TC, Rajotte RV, Larsen CP. Long-term survival of neonatal porcine islets in non-human primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. - PubMed
-
- Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transplant Immunol. 2009;21:81–86. - PubMed
-
- Rogers SA, Chen F, Talcott M, Hammerman MR. Islet cell engraftment and control of diabetes in rats following transplantation of pig pancreatic anlagen. Am J Physiol. 2004;286:E502–E509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

