Cytoskeletal regulation of epithelial barrier function during inflammation
- PMID: 20581053
- PMCID: PMC2913378
- DOI: 10.2353/ajpath.2010.100168
Cytoskeletal regulation of epithelial barrier function during inflammation
Abstract
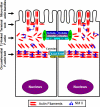
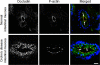
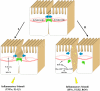
Increased epithelial permeability is a common and important consequence of mucosal inflammation that results in perturbed body homeostasis and enhanced exposure to external pathogens. The integrity and barrier properties of epithelial layers are regulated by specialized adhesive plasma membrane structures known as intercellular junctions. It is generally believed that inflammatory stimuli increase transepithelial permeability by inducing junctional disassembly. This review highlights molecular events that lead to disruption of epithelial junctions during inflammation. We specifically focus on key mechanisms of junctional regulation that are dependent on reorganization of the perijunctional F-actin cytoskeleton. We discuss critical roles of myosin-II-dependent contractility and actin filament turnover in remodeling of the F-actin cytoskeleton that drive disruption of epithelial barriers under different inflammatory conditions. Finally, we highlight signaling pathways induced by inflammatory mediators that regulate reorganization of actin filaments and junctional disassembly in mucosal epithelia.
Figures

References
-
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. - PubMed
-
- Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. - PubMed
-
- Bretscher A. Microfilament structure and function in the cortical cytoskeleton. Annu Rev Cell Biol. 1991;7:337–374. - PubMed
-
- Mooseker MS. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

