Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice
- PMID: 20581061
- PMCID: PMC2913373
- DOI: 10.2353/ajpath.2010.091287
Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice
Abstract
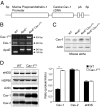
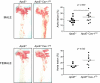
Caveolin-1 (Cav-1) is the major structural protein essential to the formation of the caveolae in endothelial cells. Genetic ablation of Cav-1 on an apolipoprotein E knockout background inhibits the progression of atherosclerosis, whereas re-expression of Cav-1 in the endothelium promotes lesion expansion. Although Cav-1-null mice are useful to delineate the importance of caveolae in atherosclerosis, there are additional problems that are difficult to dissect because loss of Cav-1 abolishes both the caveolae organelle as well as the Cav-1-mediated signaling pathways. To study how Cav-1 influences the progression of atherosclerosis in mice with caveolae, we generated a transgenic mouse that overexpresses Cav-1 in the endothelial cells in an apolipoprotein E-deficient background. We found that endothelial-specific overexpression of Cav-1 enhanced the progression of atherosclerosis in mice. Mechanistically, overexpression of Cav-1 reduced endothelial cell proliferation, migration, and nitric oxide production in vitro and increased expression of vascular cell adhesion molecule-1 in vivo.
Figures



Comment in
-
Endothelial caveolae and caveolin-1 as key regulators of atherosclerosis.Am J Pathol. 2010 Aug;177(2):544-6. doi: 10.2353/ajpath.2010.100247. Epub 2010 Jun 25. Am J Pathol. 2010. PMID: 20581059 Free PMC article.
Similar articles
-
Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis.Cell Metab. 2009 Jul;10(1):48-54. doi: 10.1016/j.cmet.2009.06.003. Cell Metab. 2009. PMID: 19583953 Free PMC article.
-
PPARgamma1-induced caveolin-1 enhances cholesterol efflux and attenuates atherosclerosis in apolipoprotein E-deficient mice.J Vasc Res. 2010;47(1):69-79. doi: 10.1159/000235927. Epub 2009 Sep 3. J Vasc Res. 2010. PMID: 19729954
-
Genetic ablation of caveolin-1 confers protection against atherosclerosis.Arterioscler Thromb Vasc Biol. 2004 Jan;24(1):98-105. doi: 10.1161/01.ATV.0000101182.89118.E5. Epub 2003 Oct 16. Arterioscler Thromb Vasc Biol. 2004. PMID: 14563650
-
Caveolae and caveolin-1: novel potential targets for the treatment of cardiovascular disease.Curr Pharm Des. 2007;13(17):1761-9. doi: 10.2174/138161207780831202. Curr Pharm Des. 2007. PMID: 17584106 Review.
-
Getting rid of caveolins: phenotypes of caveolin-deficient animals.Biochim Biophys Acta. 2005 Dec 30;1746(3):322-33. doi: 10.1016/j.bbamcr.2005.06.001. Epub 2005 Jun 23. Biochim Biophys Acta. 2005. PMID: 16019085 Review.
Cited by
-
Caveolae, caveolins, cavins, and endothelial cell function: new insights.Front Physiol. 2012 Jan 6;2:120. doi: 10.3389/fphys.2011.00120. eCollection 2012. Front Physiol. 2012. PMID: 22232608 Free PMC article.
-
Genetic variants on chromosomes 7p31 and 12p12 are associated with abnormal atrial electrical activation in patients with early-onset lone atrial fibrillation.Ann Noninvasive Electrocardiol. 2019 Nov;24(6):e12661. doi: 10.1111/anec.12661. Epub 2019 Jun 1. Ann Noninvasive Electrocardiol. 2019. PMID: 31152482 Free PMC article.
-
LRP6 protein regulates low density lipoprotein (LDL) receptor-mediated LDL uptake.J Biol Chem. 2012 Jan 6;287(2):1335-44. doi: 10.1074/jbc.M111.295287. Epub 2011 Nov 28. J Biol Chem. 2012. PMID: 22128165 Free PMC article.
-
Gliclazide Prevents 5-FU-Induced Oral Mucositis by Reducing Oxidative Stress, Inflammation, and P-Selectin Adhesion Molecules.Front Physiol. 2019 Mar 26;10:327. doi: 10.3389/fphys.2019.00327. eCollection 2019. Front Physiol. 2019. PMID: 30971955 Free PMC article.
-
Regulation of Cell Signaling and Function by Endothelial Caveolins: Implications in Disease.Transl Med (Sunnyvale). 2012;Suppl 8:001. doi: 10.4172/2161-1025.S8-001. Epub 2012 Jan 4. Transl Med (Sunnyvale). 2012. PMID: 26605130 Free PMC article.
References
-
- Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. - PubMed
-
- Busse R, Fleming I. Endothelial dysfunction in atherosclerosis. J Vasc Res. 1996;33:181–194. - PubMed
-
- Dimmeler S, Hermann C, Zeiher AM. Apoptosis of endothelial cells. Contribution to the pathophysiology of atherosclerosis? Eur Cytokine Netw. 1998;9:697–698. - PubMed
-
- Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

