Electrophysiological studies of transgenic long QT type 1 and type 2 rabbits reveal genotype-specific differences in ventricular refractoriness and His conduction
- PMID: 20581090
- PMCID: PMC2944497
- DOI: 10.1152/ajpheart.00074.2010
Electrophysiological studies of transgenic long QT type 1 and type 2 rabbits reveal genotype-specific differences in ventricular refractoriness and His conduction
Abstract
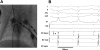
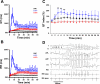
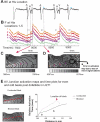
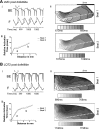
We have generated transgenic rabbits lacking cardiac slow delayed-rectifier K(+) current [I(Ks); long QT syndrome type 1 (LQT1)] or rapidly activating delayed-rectifier K(+) current [I(Kr); long QT syndrome type 2 (LQT2)]. Rabbits with either genotype have prolonged action potential duration and QT intervals; however, only LQT2 rabbits develop atrioventricular (AV) blocks and polymorphic ventricular tachycardia. We therefore sought to characterize the genotype-specific differences in AV conduction and ventricular refractoriness in LQT1 and LQT2 rabbits. We carried out in vivo electrophysiological studies in LQT1, LQT2, and littermate control (LMC) rabbits at baseline, during isoproterenol infusion, and after a bolus of dofetilide and ex vivo optical mapping studies of the AV node/His-region at baseline and during dofetilide perfusion. Under isoflurane anesthesia, LQT2 rabbits developed infra-His blocks, decremental His conduction, and prolongation of the Wenckebach cycle length. In LQT1 rabbits, dofetilide altered the His morphology and slowed His conduction, resulting in intra-His block, and additionally prolonged the ventricular refractoriness, leading to pseudo-AV block. The ventricular effective refractory period (VERP) in right ventricular apex and base was significantly longer in LQT2 than LQT1 (P < 0.05) or LMC (P < 0.01), with a greater VERP dispersion in LQT2 than LQT1 rabbits. Isoproterenol reduced the VERP dispersion in LQT2 rabbits by shortening the VERP in the base more than in the apex but had no effect on VERP in LQT1. EPS and optical mapping experiments demonstrated genotype-specific differences in AV conduction and ventricular refractoriness. The occurrence of infra-His blocks in LQT2 rabbits under isoflurane and intra-His block in LQT1 rabbits after dofetilide suggest differential regional sensitivities of the rabbit His-Purkinje system to drugs blocking I(Kr) and I(Ks).
Figures




References
-
- Ben Caref E, Boutjdir M, Himel HD, El-Sherif N. Role of subendocardial Purkinje network in triggering torsade de pointes arrhythmia in experimental long-QT syndrome. Europace 10: 1218–1223, 2008 - PubMed
-
- Bhandari AK, Shapiro WA, Morady F, Shen EN, Mason J, Scheinman MM. Electrophysiologic testing in patients with the long-QT syndrome. Circulation 71: 63–71, 1985 - PubMed
-
- Brunner M, Guo W, Mitchell GF, Buckett PD, Nerbonne JM, Koren G. Characterization of mice with a combined suppression of Ito and IK,slow. Am J Physiol Heart Circ Physiol 281: H1201–H1209, 2001 - PubMed
-
- Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, Mathur R, Hajjiri M, Odening KE, Steinberg E, Folco E, Pringa E, Centracchio J, Macharzina R, Donahay T, Schofield L, Rana N, Kirk M, Mitchel G, Poppas A, Zehender M, Koren G. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long-QT syndrome. J Clin Invest 118: 2246–2259, 2008 - PMC - PubMed
-
- Buxton AE, Josephson ME. Role of electrophysiologic studies in identifying arrhythmogenic properties of antiarrhythmic drugs. Circulation 73: II-67–II-72, 1986 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

