Characterization of N-linked protein glycosylation in Helicobacter pullorum
- PMID: 20581208
- PMCID: PMC2944503
- DOI: 10.1128/JB.00211-10
Characterization of N-linked protein glycosylation in Helicobacter pullorum
Abstract
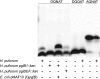
The first bacterial N-linked glycosylation system was discovered in Campylobacter jejuni, and the key enzyme involved in the coupling of glycan to asparagine residues within the acceptor sequon of the glycoprotein is the oligosaccharyltransferase PglB. Emerging genome sequence data have revealed that pglB orthologues are present in a subset of species from the Deltaproteobacteria and Epsilonproteobacteria, including three Helicobacter species: H. pullorum, H. canadensis, and H. winghamensis. In contrast to C. jejuni, in which a single pglB gene is located within a larger gene cluster encoding the enzymes required for the biosynthesis of the N-linked glycan, these Helicobacter species contain two unrelated pglB genes (pglB1 and pglB2), neither of which is located within a larger locus involved in protein glycosylation. In complementation experiments, the H. pullorum PglB1 protein, but not PglB2, was able to transfer C. jejuni N-linked glycan onto an acceptor protein in Escherichia coli. Analysis of the characterized C. jejuni N-glycosylation system with an in vitro oligosaccharyltransferase assay followed by matrix-assisted laser desorption ionization (MALDI) mass spectrometry demonstrated the utility of this approach, and when applied to H. pullorum, PglB1-dependent N glycosylation with a linear pentasaccharide was observed. This reaction required an acidic residue at the -2 position of the N-glycosylation sequon, as for C. jejuni. Attempted insertional knockout mutagenesis of the H. pullorum pglB2 gene was unsuccessful, suggesting that it is essential. These first data on N-linked glycosylation in a second bacterial species demonstrate the similarities to, and fundamental differences from, the well-studied C. jejuni system.
Figures


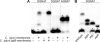

References
-
- Baar, C., M. Eppinger, G. Raddatz, J. Simon, C. Lanz, O. Klimmek, R. Nandakumar, R. Gross, A. Rosinus, H. Keller, B. Linke, F. Meyer, H. Lederer, and S. C. Schuster. 2003. Complete genome sequence and analysis of Wolinella succinogenes. Proc. Natl. Acad. Sci. U. S. A. 100:11690-11695. - PMC - PubMed
-
- Campbell, B. J., A. S. Engel, M. L. Porter, and K. Takai. 2006. The versatile ɛ-proteobacteria: key players in sulphidic habitats. Nat. Rev. Microbiol. 4:458-468. - PubMed
-
- Chavan, M., and W. Lennarz. 2006. The molecular basis of coupling of translocation and N-glycosylation. Trends Biochem. Sci. 31:7-10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F009496/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F009321/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BBC5196701/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- B19088/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

