Glial cells and chronic pain
- PMID: 20581331
- PMCID: PMC3017463
- DOI: 10.1177/1073858409360822
Glial cells and chronic pain
Abstract
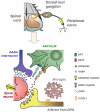
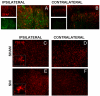
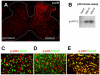
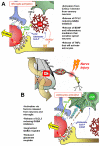
Over the past few years, the control of pain exerted by glial cells has emerged as a promising target against pathological pain. Indeed, changes in glial phenotypes have been reported throughout the entire nociceptive pathway, from peripheral nerves to higher integrative brain regions, and pharmacological inhibition of such glial reactions reduces the manifestation of pain in animal models. This complex interplay between glia and neurons relies on various mechanisms depending both on glial cell types considered (astrocytes, microglia, satellite cells, or Schwann cells), the anatomical location of the regulatory process (peripheral nerve, spinal cord, or brain), and the nature of the chronic pain paradigm. Intracellularly, recent advances have pointed to the activation of specific cascades, such as mitogen-associated protein kinases (MAPKs) in the underlying processes behind glial activation. In addition, given the large number of functions accomplished by glial cells, various mechanisms might sensitize nociceptive neurons including a release of pronociceptive cytokines and neurotrophins or changes in neurotransmitter-scavenging capacity. The authors review the conceptual advances made in the recent years about the implication of central and peripheral glia in animal models of chronic pain and discuss the possibility to translate it into human therapies in the future.
Figures
Similar articles
-
Perspectives in Pain Research 2014: Neuroinflammation and glial cell activation: The cause of transition from acute to chronic pain?Scand J Pain. 2015 Jan 1;6(1):3-6. doi: 10.1016/j.sjpain.2014.10.002. Scand J Pain. 2015. PMID: 29911589
-
Glia and pain: is chronic pain a gliopathy?Pain. 2013 Dec;154 Suppl 1(0 1):S10-S28. doi: 10.1016/j.pain.2013.06.022. Epub 2013 Jun 20. Pain. 2013. PMID: 23792284 Free PMC article. Review.
-
The induction of pain: an integrative review.Prog Neurobiol. 1999 Jan;57(1):1-164. doi: 10.1016/s0301-0082(98)00048-3. Prog Neurobiol. 1999. PMID: 9987804 Review.
-
Astrocytes in Chronic Pain: Cellular and Molecular Mechanisms.Neurosci Bull. 2023 Mar;39(3):425-439. doi: 10.1007/s12264-022-00961-3. Epub 2022 Nov 14. Neurosci Bull. 2023. PMID: 36376699 Free PMC article. Review.
-
Techniques and Methods of Animal Brain Surgery: Perfusion, Brain Removal, and Histological Techniques.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 15. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 15. PMID: 26269921 Free Books & Documents. Review.
Cited by
-
New perspectives in the diagnosis and management of enteric neuropathies.Nat Rev Gastroenterol Hepatol. 2013 Apr;10(4):206-18. doi: 10.1038/nrgastro.2013.18. Epub 2013 Feb 12. Nat Rev Gastroenterol Hepatol. 2013. PMID: 23399525 Review.
-
Crosstalk between astrocytic CXCL12 and microglial CXCR4 contributes to the development of neuropathic pain.Mol Pain. 2016 Mar 8;12:1744806916636385. doi: 10.1177/1744806916636385. Print 2016. Mol Pain. 2016. PMID: 27030717 Free PMC article.
-
Percept of the duration of a vibrotactile stimulus is altered by changing its amplitude.Front Syst Neurosci. 2015 May 21;9:77. doi: 10.3389/fnsys.2015.00077. eCollection 2015. Front Syst Neurosci. 2015. PMID: 26052273 Free PMC article.
-
Exchange proteins directly activated by cyclic adenosine monophosphate inhibitor reverses mechanical allodynia via the modification of astrocytes activity in the spinal cord.Ann Transl Med. 2021 Nov;9(22):1656. doi: 10.21037/atm-21-5384. Ann Transl Med. 2021. PMID: 34988165 Free PMC article.
-
Perioperative nerve blockade: clues from the bench.Anesthesiol Res Pract. 2011;2011:124898. doi: 10.1155/2011/124898. Epub 2011 Jul 12. Anesthesiol Res Pract. 2011. PMID: 21776253 Free PMC article.
References
-
- Brisby H, Olmarker K, Rosengren L, Cederlund CG, Rydevik B. Markers of nerve tissue injury in the cerebrospinal fluid in patients with lumbar disc herniation and sciatica. Spine. 1999;24(8):742–6. - PubMed
-
- Chang YW, Tan A, Saab C, Waxman S. Unilateral Focal Burn Injury Is Followed by Long-Lasting Bilateral Allodynia and Neuronal Hyperexcitability in Spinal Cord Dorsal Horn. J Pain. 2009 - PubMed
-
- Chudler EH, Anderson LC, Byers MR. Trigeminal ganglion neuronal activity and glial fibrillary acidic protein immunoreactivity after inferior alveolar nerve crush in the adult rat. Pain. 1997;73(2):141–9. - PubMed
-
- Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 2007;11(2):223–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

