A phase I dose-escalation study of edotecarin (J-107088) combined with infusional 5-fluorouracil and leucovorin in patients with advanced/metastatic solid tumors
- PMID: 20581657
- PMCID: PMC2931818
- DOI: 10.1097/CAD.0b013e32833cb658
A phase I dose-escalation study of edotecarin (J-107088) combined with infusional 5-fluorouracil and leucovorin in patients with advanced/metastatic solid tumors
Abstract
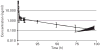
Edotecarin (J-107088), a novel inhibitor of topoisomerase I has an additive effect on colon cell lines (HCT-116) when combined with 5-fluorouracil (5-FU). We conducted a phase I study to determine the maximum tolerated dose and recommended a phase II dose of edotecarin in combination with infusional 5-FU/leucovorin (LV) in patients with advanced solid tumors. Patients and cohorts of three to six patients were sequentially enrolled at progressively higher dose levels of edotecarin administered as a 1-h intravenous (IV) infusion every 2 weeks. The edotecarin starting dose was 6 mg/m, followed by 200 mg/m LV IV infusion administered over 2 h, then 400 mg/m bolus dose of 5-FU before the start of 2400 mg/m 5-FU continuous infusion for a further 46 h. Patients were evaluated for safety, pharmacokinetics, and tumor response according to the Response Evaluation Criteria in Solid Tumors criteria. Fourteen patients (10 male; four female) received a total of 90 cycles (range 3-18). Dose-limiting toxicities were observed in five of the 14 patients treated in the study. All dose-limiting toxicities were related to neutropenia. Only the 6 and 8 mg/m edotecarin dose levels were explored; however, no maximum tolerated dose was declared. One confirmed complete response in a patient with hepatocellular carcinoma and seven stable disease responses were achieved in the 14 treated patients. Pharmacokinetic analysis showed that edotecarin achieved and maintained apparent steady-state plasma concentrations during the IV administration in both the cycles. The administration of edotecarin in combination with infusional 5-FU/LV once every 14 days, even without the 5-FU bolus, did not permit adequate time for recovery from neutropenia.
Figures

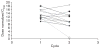

References
-
- Saif MW, Diasio RB. Edotecarin: a novel topoisomerase I inhibitor. Clin Colorectal Cancer. 2005;5:27–36. - PubMed
-
- Ciomei M, Croci V, Ciavolella A, Ballinari D, Pesenti E. Antitumor efficacy of edotecarin as a single agent and in combination with chemotherapy agents in a xenograft model. Clin Cancer Res. 2006;12:2856–2861. - PubMed
-
- Ciomei M, Croci V, Pesenti E, Kodera T, Monden Y, Ciavolella A, et al. Antitumor efficacy of edotecarin in combination with other cytotoxic drugs. Proc Amer Assoc Cancer Res. 2004:45. Abstract # 2099.
-
- Cavazos CM, Keir ST, Yoshinari T, Bigner DD, Friedman HS. Therapeutic activity of the topoisomerase I inhibitor J-107088 [6–N-(1-hydroxymethyla-2-hydroxyl) ethylamino-12,13-dihydro-13-(beta-D-glucopyranosyl)-5Hindolo( 2,3-a)-pyrrolo(3,4-c)-carbazole-5,7(6H)-dione] against pediatric and adult central nervous system tumor xenografts. Cancer Chemother Pharmacol. 2001;48:250–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

