Single-cell NF-kappaB dynamics reveal digital activation and analogue information processing
- PMID: 20581820
- PMCID: PMC3105528
- DOI: 10.1038/nature09145
Single-cell NF-kappaB dynamics reveal digital activation and analogue information processing
Abstract
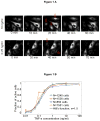
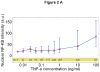
Cells operate in dynamic environments using extraordinary communication capabilities that emerge from the interactions of genetic circuitry. The mammalian immune response is a striking example of the coordination of different cell types. Cell-to-cell communication is primarily mediated by signalling molecules that form spatiotemporal concentration gradients, requiring cells to respond to a wide range of signal intensities. Here we use high-throughput microfluidic cell culture and fluorescence microscopy, quantitative gene expression analysis and mathematical modelling to investigate how single mammalian cells respond to different concentrations of the signalling molecule tumour-necrosis factor (TNF)-alpha, and relay information to the gene expression programs by means of the transcription factor nuclear factor (NF)-kappaB. We measured NF-kappaB activity in thousands of live cells under TNF-alpha doses covering four orders of magnitude. We find, in contrast to population-level studies with bulk assays, that the activation is heterogeneous and is a digital process at the single-cell level with fewer cells responding at lower doses. Cells also encode a subtle set of analogue parameters to modulate the outcome; these parameters include NF-kappaB peak intensity, response time and number of oscillations. We developed a stochastic mathematical model that reproduces both the digital and analogue dynamics as well as most gene expression profiles at all measured conditions, constituting a broadly applicable model for TNF-alpha-induced NF-kappaB signalling in various types of cells. These results highlight the value of high-throughput quantitative measurements with single-cell resolution in understanding how biological systems operate.
Figures










References
-
- Alberts B, et al. The Molecular Biology of the Cell. 5. Chap 25. Garland Science; Abington, UK: 2008. p. 1539.
-
- Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U. Dynamics of the p53-mdm2 feedback loop in individual cells. Nature Genetics. 2004;36:147. - PubMed
-
- Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

