The Ras oncogene signals centrosome amplification in mammary epithelial cells through cyclin D1/Cdk4 and Nek2
- PMID: 20581865
- PMCID: PMC2972189
- DOI: 10.1038/onc.2010.253
The Ras oncogene signals centrosome amplification in mammary epithelial cells through cyclin D1/Cdk4 and Nek2
Abstract
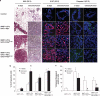
Centrosome amplification (CA) contributes to carcinogenesis by generating aneuploidy. Elevated frequencies of CA in most benign breast lesions and primary tumors suggest a causative role for CA in breast cancers. Clearly, identifying which and how altered signal transduction pathways contribute to CA is crucial to breast cancer control. Although a causative and cooperative role for c-Myc and Ras in mammary tumorigenesis is well documented, their ability to generate CA during mammary tumor initiation remains unexplored. To answer that question, K-Ras(G12D) and c-Myc were induced in mouse mammary glands. Although CA was observed in mammary tumors initiated by c-Myc or K-Ras(G12D), it was detected only in premalignant mammary lesions expressing K-Ras(G12D). CA, both in vivo and in vitro, was associated with increased expression of the centrosome-regulatory proteins, cyclin D1 and Nek2. Abolishing the expression of cyclin D1, Cdk4 or Nek2 in MCF10A human mammary epithelial cells expressing H-Ras(G12V) abrogated Ras-induced CA, whereas silencing cyclin E1 or B2 had no effect. Thus, we conclude that CA precedes mammary tumorigenesis, and interfering with centrosome-regulatory targets suppresses CA.
Figures



References
-
- Bailly E, Pines J, Hunter T, Bornens M. Cytoplasmic accumulation of cyclin B1 in human cells: association with a detergent-resistant compartment and with the centrosome. J Cell Sci. 1992;101(Part 3):529–545. - PubMed
-
- Bearss DJ, Lee RJ, Troyer DA, Pestell RG, Windle JJ. Differential effects of p21(WAF1/CIP1) deficiency on MMTV-ras and MMTV-myc mammary tumor properties. Cancer Res. 2002;62:2077–2084. - PubMed
-
- Berman H, Zhang J, Crawford YG, Gauthier ML, Fordyce CA, McDermott KM, et al. Genetic and epigenetic changes in mammary epithelial cells identify a subpopulation of cells involved in early carcinogenesis. Cold Spring Harb Symp Quant Biol. 2005;70:317–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HD047470/HD/NICHD NIH HHS/United States
- P01 CA097189/CA/NCI NIH HHS/United States
- R01 CA085619/CA/NCI NIH HHS/United States
- R01 CA098371/CA/NCI NIH HHS/United States
- R01CA98371/CA/NCI NIH HHS/United States
- R01CA85619/CA/NCI NIH HHS/United States
- R01 CA121275/CA/NCI NIH HHS/United States
- K01CA104079/CA/NCI NIH HHS/United States
- R01CA121275/CA/NCI NIH HHS/United States
- R01 CA151521/CA/NCI NIH HHS/United States
- T32 GM008490/GM/NIGMS NIH HHS/United States
- R01 HD042619/HD/NICHD NIH HHS/United States
- R01 HD047470/HD/NICHD NIH HHS/United States
- K01 CA104079/CA/NCI NIH HHS/United States
- R01HD042619/HD/NICHD NIH HHS/United States
- P01CA097189/CA/NCI NIH HHS/United States
- U01 CA105490/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

