H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3
- PMID: 20581867
- PMCID: PMC2945381
- DOI: 10.1038/onc.2010.257
H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3
Abstract
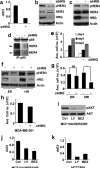
Hyperactivation of phosphatidylinositol-3 kinase (PI3K) can occur as a result of somatic mutations in PIK3CA, the gene encoding the p110α subunit of PI3K. The HER2 oncogene is amplified in 25% of all breast cancers and some of these tumors also harbor PIK3CA mutations. We examined mechanisms by which mutant PI3K can enhance transformation and confer resistance to HER2-directed therapies. We introduced the PI3K mutations E545K and H1047R in MCF10A human mammary epithelial cells that also overexpress HER2. Both mutants conferred a gain of function to MCF10A/HER2 cells. Expression of H1047R PI3K, but not E545K PI3K, markedly upregulated the HER3/HER4 ligand heregulin (HRG). HRG siRNA inhibited growth of H1047R but not E545K-expressing cells and synergized with the HER2 inhibitors trastuzumab and lapatinib. The PI3K inhibitor BEZ235 markedly inhibited HRG and pAKT levels and, in combination with lapatinib, completely inhibited growth of cells expressing H1047R PI3K. These observations suggest that PI3K mutants enhance HER2-mediated transformation by amplifying the ligand-induced signaling output of the ErbB network. This also counteracts the full effect of therapeutic inhibitors of HER2. These data also suggest that mammary tumors that contain both HER2 gene amplification and PIK3CA mutations should be treated with a combination of HER2 and PI3K inhibitors.
Figures


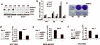
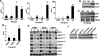

References
-
- Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, et al. The PIK3CA gene is mutated in high frequency in human breast cancers. Cancer Biol. Therapy. 2004;3:772–775. - PubMed
-
- Bellacosa A, Feo DD, Godwin AK, Bell DW, Cheng JQ, Altomare DA, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer. 1995;64:280–285. - PubMed
-
- Berns K, Horlings HM, Hennnessy BT, Mardiredjo M, Hiijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzuamb resistance in breast cancer. Cancer Cell. 2007;12:395–402. - PubMed
-
- Campbell IG, Russell SE, Choong DYH, Montgomery KG, Ciavarella ML, Hooi CSF, et al. Mutation in the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

