Immobilization of magnetic iron oxide nanoparticles on laponite discs - an easy way to biocompatible ferrofluids and ferrogels
- PMID: 20582149
- PMCID: PMC2889675
- DOI: 10.1039/c0jm00061b
Immobilization of magnetic iron oxide nanoparticles on laponite discs - an easy way to biocompatible ferrofluids and ferrogels
Abstract
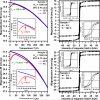
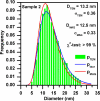
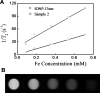
Magnetic nanocomposites containing iron oxide (maghemite) nanoparticles, well embedded in a synthetic clay matrix (laponite) were prepared by a new one step chemical route and characterized by TEM, XRD, magnetization measurements, Mössbauer spectroscopy, DLS, and MRI measurements. The synthetic procedure leads to non-stoichiometric γ-Fe(2)O(3) with a controllable content in the nanocomposite. Magnetic nanoparticles incorporated in the diamagnetic clay matrix exhibit a mean diameter of 13 nm, superparamagnetic behavior with a high saturation magnetization achievable at low applied magnetic fields. In-field Mössbauer spectra and ZFC/FC magnetization curves reveal a perfect ferrimagnetic ordering within nanoparticles with negligible spin frustration and interparticle interactions due to the complete coating of maghemite surfaces by the nanocrystalline laponite matrix. Magnetic iron oxide nanoparticles embedded in laponite matrix exhibit strong T(2) weighted MRI contrast. The maghemite/laponite composite particles have 200 nm hydrodynamic diameter and form very stable hydrosols and/or hydrogels depending on their concentration in water.
Figures






Similar articles
-
LAPONITE® nanodisk-"decorated" Fe3O4 nanoparticles: a biocompatible nano-hybrid with ultrafast magnetic hyperthermia and MRI contrast agent ability.J Mater Chem B. 2022 Jul 6;10(26):4935-4943. doi: 10.1039/d2tb00139j. J Mater Chem B. 2022. PMID: 35535802
-
One-step solid state synthesis of capped γ-Fe(2)O(3) nanocrystallites.Nanotechnology. 2008 Mar 5;19(9):095602. doi: 10.1088/0957-4484/19/9/095602. Epub 2008 Feb 12. Nanotechnology. 2008. PMID: 21817677
-
The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles.Biomaterials. 2011 Jul;32(21):4704-13. doi: 10.1016/j.biomaterials.2011.03.039. Epub 2011 Apr 19. Biomaterials. 2011. PMID: 21507482
-
Cyclo(Arg-Gly-Asp-D-Try-Glu) conjugated to ultrasmall superparamagnetic iron oxide nanoparticles.2008 Feb 16 [updated 2008 Apr 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Feb 16 [updated 2008 Apr 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641544 Free Books & Documents. Review.
-
Sialy Lewisx mimetic conjugated to ultrasmall superparamagnetic iron oxide nanoparticles.2007 Mar 5 [updated 2007 Mar 20]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Mar 5 [updated 2007 Mar 20]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22993873 Free Books & Documents. Review.
Cited by
-
Tunable Magnetic Hyperthermia Properties of Pristine and Mildly Reduced Graphene Oxide/Magnetite Nanocomposite Dispersions.Nanomaterials (Basel). 2020 Dec 4;10(12):2426. doi: 10.3390/nano10122426. Nanomaterials (Basel). 2020. PMID: 33291627 Free PMC article.
-
Chitosan-nanoclay embolic material for catheter-directed arterial embolization.J Biomed Mater Res A. 2024 Jun;112(6):914-930. doi: 10.1002/jbm.a.37670. Epub 2024 Jan 16. J Biomed Mater Res A. 2024. PMID: 38229508 Free PMC article.
-
In Situ Synthesis of Magnetic Field-Responsive Hemicellulose Hydrogels for Drug Delivery.Biomacromolecules. 2015 Aug 10;16(8):2522-8. doi: 10.1021/acs.biomac.5b00801. Epub 2015 Jul 30. Biomacromolecules. 2015. PMID: 26196600 Free PMC article.
-
Fabrication and Optimization of Stable, Optically Transparent, and Reusable pH-Responsive Silk Membranes.Int J Mol Sci. 2016 Nov 15;17(11):1897. doi: 10.3390/ijms17111897. Int J Mol Sci. 2016. PMID: 27854303 Free PMC article.
-
Maghemite Intercalated Montmorillonite as New Nanofillers for Photopolymers.Nanomaterials (Basel). 2012 Nov 19;2(4):413-427. doi: 10.3390/nano2040413. Nanomaterials (Basel). 2012. PMID: 28348316 Free PMC article.
References
-
- Yoon M, Kim YM, Kim Y, Volkov V, Song HJ, Park YJ, Vasilyak SL, Park IW. J. Magn. Magn. Mater. 2003;265:357.
-
- Teng W, Liang XY, Rahman S, Yang H. Adv. Mater. 2005;17:2237.
-
- Oliveira LCA, Petkowicz DI, Smaniotto A, Pergher SBC. Water Res. 2004;38:3699. - PubMed
-
- Wu RC, Qu HH, He H, Yu YB. Appl. Catal. B. 2004;48:49.
-
- Pankurst QA, Connoly J, Jones SK, Dobson J. J. Phys. D Appl. Phys. 2003;36:R167.
Grants and funding
LinkOut - more resources
Full Text Sources
