Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature
- PMID: 20582166
- PMCID: PMC2889818
- DOI: 10.1371/journal.pone.0011251
Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature
Abstract
Background: Pulmonary hypertension (PH) is a disease of multiple etiologies with several common pathological features, including inflammation and pulmonary vascular remodeling. Recent evidence has suggested a potential role for the recruitment of bone marrow-derived (BMD) progenitor cells to this remodeling process. We recently demonstrated that hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) is chemotactic to murine bone marrow cells in vitro and involved in pulmonary vascular remodeling in vivo.
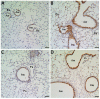
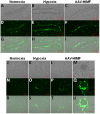
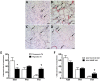
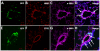
Methodology/principal findings: We used a mouse bone marrow transplant model in which lethally irradiated mice were rescued with bone marrow transplanted from green fluorescent protein (GFP)(+) transgenic mice to determine the role of HIMF in recruiting BMD cells to the lung vasculature during PH development. Exposure to chronic hypoxia and pulmonary gene transfer of HIMF were used to induce PH. Both models resulted in markedly increased numbers of BMD cells in and around the pulmonary vasculature; in several neomuscularized small (approximately 20 microm) capillary-like vessels, an entirely new medial wall was made up of these cells. We found these GFP(+) BMD cells to be positive for stem cell antigen-1 and c-kit, but negative for CD31 and CD34. Several of the GFP(+) cells that localized to the pulmonary vasculature were alpha-smooth muscle actin(+) and localized to the media layer of the vessels. This finding suggests that these cells are of mesenchymal origin and differentiate toward myofibroblast and vascular smooth muscle. Structural location in the media of small vessels suggests a functional role in the lung vasculature. To examine a potential mechanism for HIMF-dependent recruitment of mesenchymal stem cells to the pulmonary vasculature, we performed a cell migration assay using cultured human mesenchymal stem cells (HMSCs). The addition of recombinant HIMF induced migration of HMSCs in a phosphoinosotide-3-kinase-dependent manner.
Conclusions/significance: These results demonstrate HIMF-dependent recruitment of BMD mesenchymal-like cells to the remodeling pulmonary vasculature.
Conflict of interest statement
Figures



References
-
- Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–1067. - PubMed
-
- Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, et al. Hypoxia-Induced Mitogenic Factor (HIMF) Is Involved in the Pulmonary Vascular Remodeling Associated with Chronic Hypoxia and Inflammatory Asthma. American Thoracic Society International Conference 2009
-
- Su Q, Zhou Y, Johns RA. Bruton's tyrosine kinase (BTK) is a binding partner for hypoxia induced mitogenic factor (HIMF/FIZZ1) and mediates myeloid cell chemotaxis. FASEB J. 2007;21:1376–1382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

