A new stability indicating HPLC method for related substances in zolmitriptan
- PMID: 20582203
- PMCID: PMC2883213
- DOI: 10.4103/0250-474X.62252
A new stability indicating HPLC method for related substances in zolmitriptan
Abstract
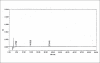
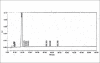
A sensitive, precise, specific, linear and stability indicating isocratic HPLC method was developed for the analysis of related substances in zolmitriptan. The potential known related substances are (S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one (impurity I) and (S)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one (impurity II). The method can be used for the detection and quantification of known and unknown impurities and degradants in the drug substance zolmitriptan during routine analysis and also for stability studies in view of its capability to separate degradation products.
Keywords: Forced degradation; HPLC; method development; related substances; zolmitriptan.
Figures


Similar articles
-
Identification, preparation and UHPLC determination of process-related impurity in zolmitriptan.J Pharm Biomed Anal. 2012 Jan 25;58:1-6. doi: 10.1016/j.jpba.2011.08.043. Epub 2011 Sep 16. J Pharm Biomed Anal. 2012. PMID: 21982522
-
Development and validation of a novel stability-indicating HPLC method for the quantitative determination of eleven related substances in ezetimibe drug substance and drug product.Talanta. 2015 Jul 1;139:67-74. doi: 10.1016/j.talanta.2015.02.039. Epub 2015 Feb 27. Talanta. 2015. PMID: 25882410
-
Development of an effective novel validated stability-indicating HPLC method for the resolution of brivaracetam stereoisomeric impurities.Chirality. 2020 Sep;32(9):1208-1219. doi: 10.1002/chir.23269. Epub 2020 Jul 13. Chirality. 2020. PMID: 32656914
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
-
Impurity Profile of Bronchodilators used in Asthma: A Critical Review.Curr Drug Discov Technol. 2018;15(4):272-304. doi: 10.2174/1570163814666170829141614. Curr Drug Discov Technol. 2018. PMID: 28875855 Review.
Cited by
-
Capillary electrophoresis methods for impurity profiling of drugs: A review of the past decade.J Pharm Anal. 2022 Feb;12(1):15-28. doi: 10.1016/j.jpha.2021.06.009. Epub 2021 Jul 2. J Pharm Anal. 2022. PMID: 35573874 Free PMC article. Review.
References
-
- Oldman AD, Smith LA, McQuay HJ, Moore RA. A systematic review of treatments for acute migraine. Pain. 2002;97:247–57. - PubMed
-
- Yates R, Nairn K, Dixon R, Seaber E. Preliminary studies of the pharmacokinetics and tolerability of zolmitriptan nasal spray in healthy volunteers. J Clin Pharmacol. 2002;42:1237–43. - PubMed
-
- Yates R, Nairn K, Dixon R, Kemp JV, Dane AL. Pharmacokinetics, dose proportionality and tolerability of single and repeat doses of a nasal spray formulation of zolmitriptan in healthy volunteers. J Clin Pharmacol. 2002;42:1244–50. - PubMed
-
- Chen X, Liu D, Luan Y, Jin F, Zhong D. Determination of zolmitriptan in human plasma by liquid chromatography-tandem mass spectrometry method: Application to a pharmacokinetic study. J Chromatogr B. 2006;832:30–5. - PubMed
-
- Yu L, Wen Y, Song Z, Mu D, Su L, Yang Y. Determination of zolmitriptan and its pharmacokinetics in human plasma after intranasal administration using LC-MS. Fenxi Ceshi Xuebao. 2006;25:67–70.
LinkOut - more resources
Full Text Sources
