Synaptic Network Activity Induces Neuronal Differentiation of Adult Hippocampal Precursor Cells through BDNF Signaling
- PMID: 20582276
- PMCID: PMC2858558
- DOI: 10.3389/neuro.22.001.2009
Synaptic Network Activity Induces Neuronal Differentiation of Adult Hippocampal Precursor Cells through BDNF Signaling
Abstract
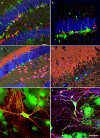
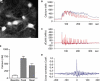
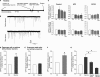
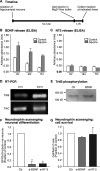
Adult hippocampal neurogenesis is regulated by activity. But how do neural precursor cells in the hippocampus respond to surrounding network activity and translate increased neural activity into a developmental program? Here we show that long-term potentiation (LTP)-like synaptic activity within a cellular network of mature hippocampal neurons promotes neuronal differentiation of newly generated cells. In co-cultures of precursor cells with primary hippocampal neurons, LTP-like synaptic plasticity induced by addition of glycine in Mg(2+)-free media for 5 min, produced synchronous network activity and subsequently increased synaptic strength between neurons. Furthermore, this synchronous network activity led to a significant increase in neuronal differentiation from the co-cultured neural precursor cells. When applied directly to precursor cells, glycine- and Mg(2+)-free solution did not induce neuronal differentiation. Synaptic plasticity-induced neuronal differentiation of precursor cells was observed in the presence of GABAergic neurotransmission blockers but was dependent on NMDA-mediated Ca(2+) influx. Most importantly, neuronal differentiation required the release of brain-derived neurotrophic factor (BDNF) from the underlying substrate hippocampal neurons as well as TrkB receptor phosphorylation in precursor cells. This suggests that activity-dependent stem cell differentiation within the hippocampal network is mediated via synaptically evoked BDNF signaling.
Keywords: adult neurogenesis; hippocampus; long-term potentiation; network oscillation; neurotrophins; precursor cell.
Figures
References
-
- Arnold F. J., Hofmann F., Bengtson C. P., Wittmann M., Vanhoutte P., Bading H. (2005). Microelectrode array recordings of cultured hippocampal networks reveal a simple model for transcription and protein synthesis-dependent plasticity. J. Physiol. 564, 3–19 10.1113/jphysiol.2004.077446 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

