Isolation and characterization of new Leptospira genotypes from patients in Mayotte (Indian Ocean)
- PMID: 20582311
- PMCID: PMC2889827
- DOI: 10.1371/journal.pntd.0000724
Isolation and characterization of new Leptospira genotypes from patients in Mayotte (Indian Ocean)
Abstract
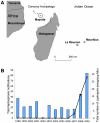
Background: Leptospirosis has been implicated as a severe and fatal form of disease in Mayotte, a French-administrated territory located in the Comoros archipelago (southwestern Indian Ocean). To date, Leptospira isolates have never been isolated in this endemic region.
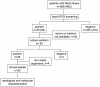
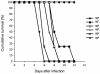
Methods and findings: Leptospires were isolated from blood samples from 22 patients with febrile illness during a 17-month period after a PCR-based screening test was positive. Strains were typed using hyper-immune antisera raised against the major Leptospira serogroups: 20 of 22 clinical isolates were assigned to serogroup Mini; the other two strains belonged to serogroups Grippotyphosa and Pyrogenes, respectively. These isolates were further characterized using partial sequencing of 16S rRNA and ligB gene, Multi Locus VNTR Analysis (MLVA), and pulsed field gel electrophoresis (PFGE). Of the 22 isolates, 14 were L. borgpetersenii strains, 7 L. kirschneri strains, and 1, belonging to serogoup Pyrogenes, was L. interrogans. Results of the genotyping methods were consistent. MLVA defined five genotypes, whereas PFGE allowed the recognition of additional subgroups within the genotypes. PFGE fingerprint patterns of clinical strains did not match any of the patterns in the reference strains belonging to the same serogroup, suggesting that the strains were novel serovars.
Conclusions: Preliminary PCR screening of blood specimen allowed a high isolation frequency of leptospires among patients with febrile illness. Typing of leptospiral isolates showed that causative agents of leptospirosis in Mayotte have unique molecular features.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–386. - PubMed
-
- Kuriakose M, Paul R, Joseph MR, Sugathan S, Sudha TN. Leptospirosis in a midland rural area of Kerala State. Indian J Med Res. 2008;128:307–312. - PubMed
-
- Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WDJ, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

