Phylogenetic and evolutionary patterns in microbial carotenoid biosynthesis are revealed by comparative genomics
- PMID: 20582313
- PMCID: PMC2889829
- DOI: 10.1371/journal.pone.0011257
Phylogenetic and evolutionary patterns in microbial carotenoid biosynthesis are revealed by comparative genomics
Abstract
Background: Carotenoids are multifunctional, taxonomically widespread and biotechnologically important pigments. Their biosynthesis serves as a model system for understanding the evolution of secondary metabolism. Microbial carotenoid diversity and evolution has hitherto been analyzed primarily from structural and biosynthetic perspectives, with the few phylogenetic analyses of microbial carotenoid biosynthetic proteins using either used limited datasets or lacking methodological rigor. Given the recent accumulation of microbial genome sequences, a reappraisal of microbial carotenoid biosynthetic diversity and evolution from the perspective of comparative genomics is warranted to validate and complement models of microbial carotenoid diversity and evolution based upon structural and biosynthetic data.
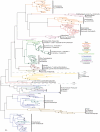
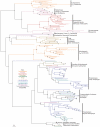
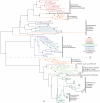
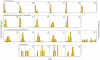
Methodology/principal findings: Comparative genomics were used to identify and analyze in silico microbial carotenoid biosynthetic pathways. Four major phylogenetic lineages of carotenoid biosynthesis are suggested composed of: (i) Proteobacteria; (ii) Firmicutes; (iii) Chlorobi, Cyanobacteria and photosynthetic eukaryotes; and (iv) Archaea, Bacteroidetes and two separate sub-lineages of Actinobacteria. Using this phylogenetic framework, specific evolutionary mechanisms are proposed for carotenoid desaturase CrtI-family enzymes and carotenoid cyclases. Several phylogenetic lineage-specific evolutionary mechanisms are also suggested, including: (i) horizontal gene transfer; (ii) gene acquisition followed by differential gene loss; (iii) co-evolution with other biochemical structures such as proteorhodopsins; and (iv) positive selection.
Conclusions/significance: Comparative genomics analyses of microbial carotenoid biosynthetic proteins indicate a much greater taxonomic diversity then that identified based on structural and biosynthetic data, and divides microbial carotenoid biosynthesis into several, well-supported phylogenetic lineages not evident previously. This phylogenetic framework is applicable to understanding the evolution of specific carotenoid biosynthetic proteins or the unique characteristics of carotenoid biosynthetic evolution in a specific phylogenetic lineage. Together, these analyses suggest a "bramble" model for microbial carotenoid biosynthesis whereby later biosynthetic steps exhibit greater evolutionary plasticity and reticulation compared to those closer to the biosynthetic "root". Structural diversification may be constrained ("trimmed") where selection is strong, but less so where selection is weaker. These analyses also highlight likely productive avenues for future research and bioprospecting by identifying both gaps in current knowledge and taxa which may particularly facilitate carotenoid diversification.
Conflict of interest statement
Figures





References
-
- Britton G, Liaaen-Jensen S, Pfander H. Basal, Switzerland: Birkhäuser Verlag; 2004. Carotenoids handbook.
-
- Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–1558. - PubMed
-
- Fraser NJ, Hashimoto H, Cogdell RJ. Carotenoids and bacterial photosynthesis: the story so far. Photosynth Res. 2001;70:249–256. - PubMed
-
- Frank HA, Brudvig GW. Redox functions of carotenoids in photosynthesis. Biochemistry. 2004;43:8607–8615. - PubMed
-
- Frank HA, Cogdell RJ. Carotenoids in photosynthesis. Photochem Photobiol. 1996;63:257–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

