Regulation of cytochrome P450 expression in Drosophila: Genomic insights
- PMID: 20582327
- PMCID: PMC2890303
- DOI: 10.1016/j.pestbp.2009.06.009
Regulation of cytochrome P450 expression in Drosophila: Genomic insights
Abstract
Genomic tools such as the availability of the Drosophila genome sequence, the relative ease of stable transformation, and DNA microarrays have made the fruit fly a powerful model in insecticide toxicology research. We have used transgenic promoter-GFP constructs to document the detailed pattern of induced Cyp6a2 gene expression in larval and adult Drosophila tissues. We also compared various insecticides and xenobiotics for their ability to induce this cytochrome P450 gene, and show that the pattern of Cyp6a2 inducibility is comparable to that of vertebrate CYP2B genes, and different from that of vertebrate CYP1A genes, suggesting a degree of evolutionary conservation for the "phenobarbital-type" induction mechanism. Our results are compared to the increasingly diverse reports on P450 induction that can be gleaned from whole genome or from "detox" microarray experiments in Drosophila. These suggest that only a third of the genomic repertoire of CYP genes is inducible by xenobiotics, and that there are distinct subsets of inducers / induced genes, suggesting multiple xenobiotic transduction mechanisms. A relationship between induction and resistance is not supported by expression data from the literature. The relative abundance of expression data now available is in contrast to the paucity of studies on functional expression of P450 enzymes, and this remains a challenge for our understanding of the toxicokinetic aspects of insecticide action.
Figures
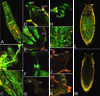
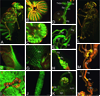
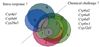
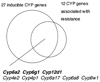
Similar articles
-
Xenobiotic response in Drosophila melanogaster: sex dependence of P450 and GST gene induction.Insect Biochem Mol Biol. 2006 Aug;36(8):674-82. doi: 10.1016/j.ibmb.2006.05.009. Epub 2006 May 25. Insect Biochem Mol Biol. 2006. PMID: 16876710
-
The Drosophila cytochrome P450 gene Cyp6a2: structure, localization, heterologous expression, and induction by phenobarbital.DNA Cell Biol. 1997 Nov;16(11):1345-56. doi: 10.1089/dna.1997.16.1345. DNA Cell Biol. 1997. PMID: 9407006
-
Induction of two cytochrome P450 genes, Cyp6a2 and Cyp6a8, of Drosophila melanogaster by caffeine in adult flies and in cell culture.Gene. 2006 Aug 1;377:56-64. doi: 10.1016/j.gene.2006.02.032. Epub 2006 Apr 5. Gene. 2006. PMID: 16713132
-
Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes.Curr Drug Metab. 2002 Oct;3(5):481-90. doi: 10.2174/1389200023337171. Curr Drug Metab. 2002. PMID: 12369894 Review.
-
Induction of the hepatic cytochrome P450 2B subfamily by xenobiotics: research history, evolutionary aspect, relation to tumorigenesis, and mechanism.Curr Drug Metab. 2006 May;7(4):397-409. doi: 10.2174/138920006776873508. Curr Drug Metab. 2006. PMID: 16724929 Review.
Cited by
-
RNA-Seq analysis of the blue light-emitting Orfelia fultoni (Diptera: Keroplatidae) suggest photoecological adaptations at the molecular level.Comp Biochem Physiol Part D Genomics Proteomics. 2021 Sep;39:100840. doi: 10.1016/j.cbd.2021.100840. Epub 2021 May 12. Comp Biochem Physiol Part D Genomics Proteomics. 2021. PMID: 34022525 Free PMC article.
-
Genome-wide patterns of gene expression during aging in the African malaria vector Anopheles gambiae.PLoS One. 2010 Oct 13;5(10):e13359. doi: 10.1371/journal.pone.0013359. PLoS One. 2010. PMID: 20967211 Free PMC article.
-
Identification and characterization of CYPs induced in the Drosophila antenna by exposure to a plant odorant.Sci Rep. 2021 Oct 15;11(1):20530. doi: 10.1038/s41598-021-99910-9. Sci Rep. 2021. PMID: 34654888 Free PMC article.
-
CYP4BN4v7 regulates the population density dependent oocyte maturity rate in bean beetles.Sci Rep. 2024 Nov 19;14(1):28574. doi: 10.1038/s41598-024-79866-2. Sci Rep. 2024. PMID: 39562601 Free PMC article.
-
Unveiling candidate genes for metabolic resistance to malathion in Aedes albopictus through RNA sequencing-based transcriptome profiling.PLoS Negl Trop Dis. 2024 Jun 12;18(6):e0012243. doi: 10.1371/journal.pntd.0012243. eCollection 2024 Jun. PLoS Negl Trop Dis. 2024. PMID: 38865422 Free PMC article.
References
-
- Welling W, Paterson GD. Toxicodynamics of insecticides. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Oxford: Pergamon Press; 1985. pp. 603–645.
-
- Wilson TG. Resistance of Drosophila to toxins. Annu Rev Entomol. 2001;46:545–571. - PubMed
-
- Wilson TG. Drosophila: Sentinels of environmental toxicants. Integr. Comp. Biol. 2005;45:127–136. - PubMed
-
- Tijet N, Helvig C, Feyereisen R. The cytochrome P450 gene superfamily in Drosophila melanogaster: annotation, intron-exon organization and phylogeny. Gene. 2001;262:189–198. - PubMed
-
- Feyereisen R. Evolution of insect P450. Biochem Soc Trans. 2006;34:1252–1255. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
