Breast cancer resistance protein (BCRP) and sulfotransferases contribute significantly to the disposition of genistein in mouse intestine
- PMID: 20582579
- PMCID: PMC2976988
- DOI: 10.1208/s12248-010-9209-x
Breast cancer resistance protein (BCRP) and sulfotransferases contribute significantly to the disposition of genistein in mouse intestine
Abstract
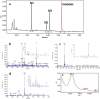
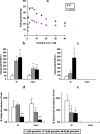
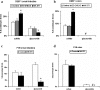
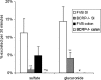
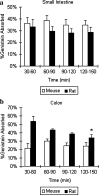
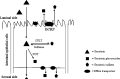
The low bioavailability of genistein has impeded its development into a therapeutic agent. Our earlier studies indicate that glucuronidation is one of the major barriers to genistein oral bioavailability. This study will determine how sulfotransferases and efflux transporters affect its intestinal disposition. A rodent intestinal perfusion model and S9 fractions were used. Sulfate excretion rates were comparable to glucuronide excretion in mouse small intestine but significantly higher than glucuronide excretion in mouse colon, which is different from rat intestinal disposition but similar to disposition in Caco-2 cells. To define efflux transporter(s) involved in sulfate excretion, two organic anion inhibitors (estrone sulfate and dihydroepiandrosterone sulfate) or a multidrug resistance protein inhibitor (MK-571) were used but neither was able to decrease the excretion of genistein sulfates. In contrast, the excretion of genistein sulfate decreased substantially (>90%) in small intestine of breast cancer resistance protein (BCRP) knockout mice and became undetectable in colon of the knockout mice. The excretion rates of genistein glucuronide in the small intestine of BCRP knockout mice were also significant decreased (78%). This study shows clearly that BCRP facilitates the cellular genistein sulfate excretion by removing sulfates to prevent their backward hydrolysis and to limit substrate inhibition, indicating that BCRP plays a dominant role in genistein sulfate excretion and a significant role in genistein glucuronide excretion in the mouse intestine.
Figures

Similar articles
-
Breast cancer resistance protein (ABCG2) determines distribution of genistein phase II metabolites: reevaluation of the roles of ABCG2 in the disposition of genistein.Drug Metab Dispos. 2012 Oct;40(10):1883-93. doi: 10.1124/dmd.111.043901. Epub 2012 Jun 26. Drug Metab Dispos. 2012. PMID: 22736306 Free PMC article.
-
Bioavailability of the glucuronide and sulfate conjugates of genistein and daidzein in breast cancer resistance protein 1 knockout mice.Drug Metab Dispos. 2011 Nov;39(11):2008-12. doi: 10.1124/dmd.111.040881. Epub 2011 Aug 9. Drug Metab Dispos. 2011. PMID: 21828252
-
Evaluation of the role of breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance-associated protein 4 (MRP4/ABCC4) in the urinary excretion of sulfate and glucuronide metabolites of edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one).Drug Metab Dispos. 2007 Nov;35(11):2045-52. doi: 10.1124/dmd.107.016352. Epub 2007 Aug 6. Drug Metab Dispos. 2007. PMID: 17682070
-
Interaction of Isoflavones with the BCRP/ABCG2 Drug Transporter.Curr Drug Metab. 2015;16(2):124-40. doi: 10.2174/138920021602150713114921. Curr Drug Metab. 2015. PMID: 26179608 Free PMC article. Review.
-
The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters.Arch Biochem Biophys. 2012 Nov 15;527(2):67-73. doi: 10.1016/j.abb.2012.06.004. Epub 2012 Jun 26. Arch Biochem Biophys. 2012. PMID: 22750234 Review.
Cited by
-
Screening and identification of glyceollins and their metabolites by electrospray ionization tandem mass spectrometry with precursor ion scanning.Anal Chem. 2013 Feb 5;85(3):1727-33. doi: 10.1021/ac3030398. Epub 2013 Jan 7. Anal Chem. 2013. PMID: 23294002 Free PMC article.
-
Glyceollin transport, metabolism, and effects on p-glycoprotein function in Caco-2 cells.J Med Food. 2014 Apr;17(4):462-71. doi: 10.1089/jmf.2013.0115. Epub 2014 Jan 29. J Med Food. 2014. PMID: 24476214 Free PMC article.
-
Genistein and Glyceollin Effects on ABCC2 (MRP2) and ABCG2 (BCRP) in Caco-2 Cells.Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010017. doi: 10.3390/ijerph13010017. Int J Environ Res Public Health. 2015. PMID: 26703673 Free PMC article.
-
Shengjiang Xiexin Decoction Alters Pharmacokinetics of Irinotecan by Regulating Metabolic Enzymes and Transporters: A Multi-Target Therapy for Alleviating the Gastrointestinal Toxicity.Front Pharmacol. 2017 Oct 27;8:769. doi: 10.3389/fphar.2017.00769. eCollection 2017. Front Pharmacol. 2017. PMID: 29163158 Free PMC article.
-
Small-molecule control of neurotransmitter sulfonation.J Biol Chem. 2021 Jan-Jun;296:100094. doi: 10.1074/jbc.RA120.015177. Epub 2020 Nov 24. J Biol Chem. 2021. PMID: 33485192 Free PMC article.
References
-
- Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–558S. - PubMed
-
- Cao F, Jin TY, Zhou YF. Inhibitory effect of isoflavones on prostate cancer cells and PTEN gene. Biomed Environ Sci. 2006;19:35–41. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

