Llama-derived single-domain antibodies for the detection of botulinum A neurotoxin
- PMID: 20582697
- PMCID: PMC3049303
- DOI: 10.1007/s00216-010-3905-3
Llama-derived single-domain antibodies for the detection of botulinum A neurotoxin
Abstract
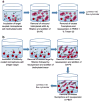
Single-domain antibodies (sdAb) specific for botulinum neurotoxin serotype A (BoNT A) were selected from an immune llama phage display library derived from a llama that was immunized with BoNT A toxoid. The constructed phage library was panned using two methods: panning on plates coated with BoNT A toxoid (BoNT A Td) and BoNT A complex toxoid (BoNT Ac Td) and panning on microspheres coupled to BoNT A Td and BoNT A toxin (BoNT A Tx). Both panning methods selected for binders that had identical sequences, suggesting that panning on toxoided material may be as effective as panning on bead-immobilized toxin for isolating specific binders. All of the isolated binders tested were observed to recognize bead-immobilized BoNT A Tx in direct binding assays, and showed very little cross-reactivity towards other BoNT serotypes and unrelated protein. Sandwich assays that incorporated selected sdAb as capture and tracer elements demonstrated that all of the sdAb were able to recognize soluble ("live") BoNT A Tx and BoNT Ac Tx with virtually no cross-reactivity with other BoNT serotypes. The isolated sdAb did not exhibit the high degree of thermal stability often associated with these reagents; after the first heating cycle most of the binding activity was lost, but the portion of the protein that did refold and recover antigen-binding activity showed only minimal loss on subsequent heating and cooling cycles. The binding kinetics of selected binders, assessed by both an equilibrium fluid array assay as well as surface plasmon resonance (SPR) using toxoided material, gave dissociation constants (K(D)) in the range 2.2 x 10(-11) to 1.6 x 10(-10) M. These high-affinity binders may prove beneficial to the development of recombinant reagents for the rapid detection of BoNT A, particularly in field screening and monitoring applications.
Figures




References
-
- Montecucco C, Molgo J. Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol. 2005;5(3):274–279. - PubMed
-
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K, Biodefense WGC. Botulinum toxin as a biological weapon—medical and public health management. JAMA. 2001;285(8):1059–1070. - PubMed
-
- Woodruff BA, Griffin PM, Mccroskey LM, Smart JF, Wainwright RB, Bryant RG, Hutwagner LC, Hatheway CL. Clinical and laboratory comparison of botulism from toxin type A, type B, and type E in the United States, 1975–1988. J Infect Dis. 1992;166(6):1281–1286. - PubMed
-
- Sharma SK, Whiting RC. Methods for detection of Clostridium botulinum toxin in foods. J Food Prot. 2005;68(6):1256–1263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

