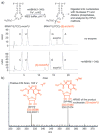
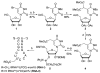
The AlkB domain of mammalian ABH8 catalyzes hydroxylation of 5-methoxycarbonylmethyluridine at the wobble position of tRNA
- PMID: 20583019
- PMCID: PMC3134247
- DOI: 10.1002/anie.201001242
The AlkB domain of mammalian ABH8 catalyzes hydroxylation of 5-methoxycarbonylmethyluridine at the wobble position of tRNA
Figures
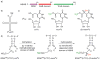
Similar articles
-
Human AlkB homolog ABH8 Is a tRNA methyltransferase required for wobble uridine modification and DNA damage survival.Mol Cell Biol. 2010 May;30(10):2449-59. doi: 10.1128/MCB.01604-09. Epub 2010 Mar 22. Mol Cell Biol. 2010. PMID: 20308323 Free PMC article.
-
Crystal structure and RNA binding properties of the RNA recognition motif (RRM) and AlkB domains in human AlkB homolog 8 (ABH8), an enzyme catalyzing tRNA hypermodification.J Biol Chem. 2012 Jan 13;287(3):2130-43. doi: 10.1074/jbc.M111.286187. Epub 2011 Nov 7. J Biol Chem. 2012. PMID: 22065580 Free PMC article.
-
Mammalian ALKBH8 possesses tRNA methyltransferase activity required for the biogenesis of multiple wobble uridine modifications implicated in translational decoding.Mol Cell Biol. 2010 Apr;30(7):1814-27. doi: 10.1128/MCB.01602-09. Epub 2010 Feb 1. Mol Cell Biol. 2010. PMID: 20123966 Free PMC article.
-
Stereochemical mechanisms of tRNA methyltransferases.FEBS Lett. 2010 Jan 21;584(2):278-86. doi: 10.1016/j.febslet.2009.11.075. FEBS Lett. 2010. PMID: 19944101 Free PMC article. Review.
-
tRNA's wobble decoding of the genome: 40 years of modification.J Mol Biol. 2007 Feb 9;366(1):1-13. doi: 10.1016/j.jmb.2006.11.046. Epub 2006 Nov 15. J Mol Biol. 2007. PMID: 17187822 Review.
Cited by
-
The expanding world of tRNA modifications and their disease relevance.Nat Rev Mol Cell Biol. 2021 Jun;22(6):375-392. doi: 10.1038/s41580-021-00342-0. Epub 2021 Mar 3. Nat Rev Mol Cell Biol. 2021. PMID: 33658722 Review.
-
Roles of Trm9- and ALKBH8-like proteins in the formation of modified wobble uridines in Arabidopsis tRNA.Nucleic Acids Res. 2011 Sep 1;39(17):7688-701. doi: 10.1093/nar/gkr406. Epub 2011 Jun 7. Nucleic Acids Res. 2011. PMID: 21653555 Free PMC article.
-
Spectroscopic and in vitro Investigations of Fe2+ /α-Ketoglutarate-Dependent Enzymes Involved in Nucleic Acid Repair and Modification.Chembiochem. 2022 Jun 3;23(11):e202100605. doi: 10.1002/cbic.202100605. Epub 2022 Feb 15. Chembiochem. 2022. PMID: 35040547 Free PMC article. Review.
-
The atomic resolution structure of human AlkB homolog 7 (ALKBH7), a key protein for programmed necrosis and fat metabolism.J Biol Chem. 2014 Oct 3;289(40):27924-36. doi: 10.1074/jbc.M114.590505. Epub 2014 Aug 13. J Biol Chem. 2014. PMID: 25122757 Free PMC article.
-
The Possible Mechanism of Physiological Adaptation to the Low-Se Diet and Its Health Risk in the Traditional Endemic Areas of Keshan Diseases.Biol Trace Elem Res. 2022 May;200(5):2069-2083. doi: 10.1007/s12011-021-02851-7. Epub 2021 Aug 8. Biol Trace Elem Res. 2022. PMID: 34365573 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

